
гавЛЮоЩЋЛьКЯЮяЕФЫЎШмвКЃЌжЛПЩФмКЌгавдЯТРызгжаЕФШєИЩжжЃКKЃЋЁЂNH4ЃЋЁЂClЃЁЂMg2ЃЋЁЂFe3ЃЋЁЂCO32ЃЁЂSO42ЃЁЂHЃЋЃЌЯжШЁШ§Зн100mLШмвКНјааШчЯТЪЕбщЃК
ЃЈ1ЃЉЕквЛЗнМгШызуСПAgNO3ШмвКгаГСЕэВњЩњ
ЃЈ2ЃЉЕкЖўЗнМгзуСПNaOHШмвКМгШШКѓЃЌЪеМЏЕНЦјЬх0.04mol
ЃЈ3ЃЉЕкШ§ЗнМгзуСПBaCl2ШмвККѓЃЌЕУИЩдяГСЕэ6.27gЃЌОзуСПбЮЫсЯДЕгЁЂИЩдяКѓЃЌГСЕэжЪСПЮЊ2.33gЁЃ
ИљОнЩЯЪіЪЕбщЃЌвдЯТЭЦВте§ШЗЕФЪЧЃЈ ЃЉ
AЃЎFe3ЃЋвЛЖЈВЛДцдкЃЌMg2ЃЋПЩФмДцдк
BЃЎ100mLШмвКжаКЌ0.01mol CO32Ѓ
CЃЎClЃвЛЖЈДцдк
DЃЎKЃЋвЛЖЈДцдк
D
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКгЩЃЈ1ЃЉПЩжЊШмвКжаCl?ЁЂCO32ЁЊЁЂSO42ЁЊШ§жжжажСЩйКЌгаЦфжавЛжжЃЛгЩЃЈ2ЃЉФмШЗЖЈКЌNH4+ЃЌЧвc(NH4+)=0.4molЁЄLЃ1ЃЌЕкЃЈ3ЃЉВНПЩвдШЗЖЈc(SO42ЁЊ)=0.1molЁЄlЃ1ЃЛc(CO32ЁЊ)=0.2molЁЄlЃ1ЃЌгЩгкКЌгаCO32ЁЊЁЂSO42ЁЊЃЌЫљвдШмвКжаПЯЖЈВЛКЌMg2+ЁЂFe3+ЃЛвђЮЊШмвКжавѕбєРызгЫљДјЕчКЩЯрЕШЃЈМДШмвКЪЧЕчжаадЕФЃЌетвВЪЧИУЬтФПвўКЌЕФаХЯЂЃЉЃЌгЩЩЯЪіЫљЧѓЕФвбжЊвѕбєРызгЕФХЈЖШПДГіЃЌЪЧВЛЪиКуЕФЃЌвђДЫШмвКжавЛЖЈДцдкK+ЃЌЧвc(K+)Ён0.2molЁЄlЃ1ЃЌCl?ВЛФмШЗЖЈЃЌЙЪDЯюе§ШЗЁЃ
ПМЕуЃКБОЬтПМВщРызгЭЦЖЯМАЯрЙиМЦЫуЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьКЃФЯЪЁШ§бЧЪаИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈAЃЉЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
вбжЊФГЫсадШмвКжаКЌгаBa2+ЁЂFe3+ЃЌдђЯТЪіРызгзщжаФмгыЩЯЪіРызгЙВДцЕФЪЧ( )ЁЃ
AЃЎCO32ЁЊЁЂClЁЊ BЃЎNO3ЁЊЁЂClЁЊ
CЃЎNO3ЁЊЁЂSO42ЁЊ DЃЎOHЁЊЁЂNO3ЁЊ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЩЯбЇЦкЦкжаПМЪдРэПЦЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
гУ18.4 molЁЄLЃ1ЕФХЈСђЫсХфжЦ100 mLХЈЖШЮЊ1 molЁЄLЃ1ЕФЯЁСђЫсЃЌЦфВйзїПЩЗжЮЊвдЯТИїВНЃК
AЃЎгУСПЭВСПШЁ5.4 mLХЈСђЫсЃЌЛКТ§ЕЙШызАгадМ50 mLеєСѓЫЎЕФЩеБРяЃЌВЂгУВЃСЇАєНСАш
BЃЎгУдМ30 mLеєСѓЫЎЃЌЗжГЩШ§ДЮЯДЕгЩеБКЭВЃСЇАєЃЌНЋЯДЕгвКЖМЕЙШыШнСПЦПжа
CЃЎНЋЯЁЪЭКѓЕФСђЫсаЁаФЕиЕЙШыШнСПЦПжа
DЃЎМьВщ100 mLШнСПЦППкЪЧЗёгаТЉвКЯжЯѓ
EЃЎНЋеєСѓЫЎжБНгМгШыШнСПЦПЃЌжСвКУцОрПЬЖШЯп1ЁЋ2 cmДІ
FЃЎИЧНєЦПШћЃЌЗДИДЕпЕЙеёЕДЃЌвЁдШШмвК
GЃЎгУНКЭЗЕЮЙмЯђШнСПЦПРяж№ЕЮМгШыеєСѓЫЎЃЌжСвКУцзюЕЭЕугыПЬЖШЯпЯрЧа
ЃЈ1ЃЉе§ШЗЕФВйзїЫГађгІИУЪЧ______________________________________________ЁЃ
ЃЈ2ЃЉНјааAВНВйзїЕФЪБКђЃЌгІИУбЁгУЕФЪЧ________(ЬюађКХ)ЁЃ
Ђй10 mLСПЭВЃЛЂк50 mLСПЭВЃЛЂл500 mLСПЭВЃЛЂм1000 mLСПЭВ
ШчЙћЖдзАгаХЈСђЫсЕФСПЭВЖСЪ§ШчЭМЫљЪОЃЌХфжЦЕФЯЁСђЫсЕФХЈЖШНЋ________(ЬюЁАЦЋИпЁБЁЂЁАЦЋЕЭЁБЛђЁАЮогАЯьЁБ)ЁЃ

ЃЈ3ЃЉНјааAВНВйзїКѓЃЌБиаы________ЃЌВХФмНјааCВНВйзїЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЩЯбЇЦкЦкжаПМЪдРэПЦЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
АЂЗќМгЕТТоГЃЪ§дМЮЊ6.02ЁС1023 molЃ1ЃЌЯТСаа№Ъіжае§ШЗЕФЪЧ( )
AЃЎ1.01ЁС105 PaЁЂ25 ЁцЪБЃЌ2.24 L Cl2жаКЌгаЕФдзгЪ§ЮЊ0.2ЁС6.02ЁС1023
BЃЎ0.1 L 3 molЁЄLЃ1 NH4NO3ШмвКжаКЌгаЕФNдзгЪ§ФПЮЊ0.3ЁС6.02ЁС1023
CЃЎ5.6 gЬњЗлгызуСПCuSO4ШмвКЗДгІЩњГЩЕФЭдзгЪ§ЮЊ1ЁС6.02ЁС1023
DЃЎ46 g NO2КЭN2O4ЕФЛьКЯЮяжаКЌгаЕФдзгЪ§ЮЊ3ЁС6.02ЁС1023
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЪЕбщЬт
ЯТЭМЪЧЪЕбщЪвжЦБИТШЦјВЂНјаавЛЯЕСаЯрЙиЪЕбщЕФзАжУЃЈМаГжМАМгШШвЧЦївбТдЃЉЁЃ
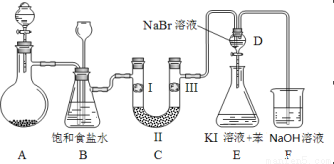
ЃЈ1ЃЉжЦБИТШЦјбЁгУЕФвЉЦЗЮЊЙЬЬхЖўбѕЛЏУЬКЭХЈбЮЫсЃЌдђЯрЙиЕФРызгЗДгІЗНГЬЪНЮЊ ЃЛ
зАжУBжаБЅКЭЪГбЮЫЎЕФзїгУЪЧ_________ЃЛЭЌЪБзАжУBврЪЧАВШЋЦПЃЌМрВтЪЕбщНјааЪБCжаЪЧЗёЗЂЩњЖТШћЃЌЧыаДГіЗЂЩњЖТШћЪБBжаЕФЯжЯѓ ЃЛ
ЃЈ2ЃЉзАжУCЕФЪЕбщФПЕФЪЧбщжЄТШЦјЪЧЗёОпгаЦЏАзадЃЌЮЊДЫCжаIЁЂIIЁЂIIIвРДЮЗХШы ЃЛ
| a | b | c | d |
I | ИЩдяЕФгаЩЋВМЬѕ | ИЩдяЕФгаЩЋВМЬѕ | ЪЊШѓЕФгаЩЋВМЬѕ | ЪЊШѓЕФгаЩЋВМЬѕ |
II | МюЪЏЛв | ЙшНК | ХЈСђЫс | ЮоЫЎТШЛЏИЦ |
III | ЪЊШѓЕФгаЩЋВМЬѕ | ЪЊШѓЕФгаЩЋВМЬѕ | ИЩдяЕФгаЩЋВМЬѕ | ИЩдяЕФгаЩЋВМЬѕ |
ЃЈ3ЃЉЩшМЦзАжУDЁЂEЕФФПЕФЪЧБШНЯТШЁЂфхЁЂЕтЕЅжЪЕФбѕЛЏадЧПШѕЁЃЕБЯђDжаЛКЛКЭЈШыЩйСПТШЦјЪБЃЌПЩвдПДЕНЮоЩЋШмвКж№НЅБфЮЊ__________ЩЋЃЌЫЕУї ЃЛ
ДђПЊЛюШћЃЌНЋзАжУDжаЩйСПШмвКМгШызАжУEжаЃЌеёЕДЁЃЙлВьЕНЕФЯжЯѓЪЧ ЃЛ
ЃЈ4ЃЉзАжУFжагУзуСПЕФNaOHШмвКЮќЪегрТШЃЌЪдаДГіЯргІЕФРызгЗНГЬЪН ЃЛ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
Rn+РызггаmИіЕчзгЃЌЫќЕФжЪСПЪ§ЮЊAЃЌдђдзгКЫФкЕФжазгЪ§ЮЊЃЈ ЃЉ
A.m +n B.AЃm+n C.AЃmЃn D.AЃЋmЃn
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаЪЕбщВйзїжае§ШЗЕФЪЧЃЈ ЃЉ
AЃЎеєСѓвКЬхЪБЃЌРфЫЎгІДгРфФ§ЙмЕФЩЯЖЫНјШы
BЃЎЙ§ТЫЪБВЃСЇАєЕФФЉЖЫгІЧсЧсППдкШ§ВуЕФТЫжНЩЯ
CЃЎЬсШЁЕтЫЎжаЕФЕтЃКМгШыЪЪСПввДМЃЌеёЕДЁЂОВжУЁЂЗжвК
DЃЎзібцЩЋЗДгІЧАгІЯШНЋВЌЫПдкЯЁСђЫсжаЯДИЩОЛдйеКШЁД§ВтвКНјааЪЕбщ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЬхЛ§ЮЊV mLЃЌУмЖШЮЊІб g/mLЕФКЌгаЯрЖдЗжзгжЪСПЮЊMЕФФГЮяжЪЕФШмвКЃЌЦфШмжЪЮЊm g,ЦфЮяжЪЕФСПХЈЖШЮЊc mol/L,ШмжЪЕФжЪСПЗжЪ§ЮЊІи%,дђЯТСаБэЪОе§ШЗЕФЪЧ
AЃЎ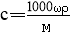 BЃЎ
BЃЎ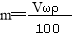 CЃЎ
CЃЎ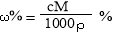 DЃЎ
DЃЎ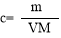
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2016НьеуНЪЁКўжнЪаЪєОХаЃИпвЛ12дТСЊПМЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
2KMnO4+16HCl=2MnCl2+5Cl2Ёќ+8H2O+2KClЃЌдкетИіЗДгІжаЃЌбѕЛЏМСЪЧ ЃЌбѕЛЏВњЮяЪЧ ЃЌгУЫЋЯпЧХБъГіЕчзгЕФзЊвЦЗНЯђКЭЪ§ФП ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com