
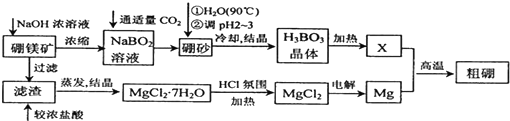
·ÖĪö ÅšĆ¾æóÖ÷ŅŖ³É·ÖĪŖMg2B2O5•H2O£¬ÅšÉ°µÄ»ÆѧŹ½ĪŖNa2B4O7•10H2O£®ĄūÓĆÅšĆ¾æóÖĘČ”½šŹōĆ¾¼°“ÖÅšµÄ¹¤ŅÕĮ÷³ĢÖŠÅšĆ¾æó¼ÓČėĒāŃõ»ÆÄĘÅØČÜŅŗ¹żĀĖµĆµ½ĀČ»ÆĆ¾£¬¼ÓČėÅØŃĪĖįČܽāĶعżÕō·¢ÅØĖõµĆµ½ĀČ»ÆĆ¾½į¾§Ė®ŗĻĪļ£¬ŌŚĀČ»ÆĒāĘųĮ÷ÖŠ¼ÓČȵƵ½ĀČ»ÆĆ¾¹ĢĢ壬¹żµē½āµĆµ½Ć¾£»ĀĖŅŗÖŠÖ÷ŅŖŹĒNaBO2£¬ĶØČėŹŹĮ涞Ńõ»ÆĢ¼ĘųĢåµĆµ½ÅšÉ°£¬ČÜÓŚČČĖ®ŗó£¬ÓĆH2SO4µ÷pH2”«3ÖĘČ”H3BO3£¬¼ÓČȵƵ½B2O3£»
£Ø1£©ÓĆH2SO4µ÷pH2”«3£¬ÅšÉ°ÖŠµÄNa2B4O7ŌŚĖįČÜŅŗÖŠÉś³ÉH3BO3 £¬XĪŖH3BO3¾§Ģå¼ÓČČĶŃĖ®µÄ²śĪļÅŠ¶ĻĪŖB2O3£¬ÓėMg·“Ӧɜ³É“ÖÅšŗĶŃõ»ÆĆ¾£»
£Ø2£©ĀČ»ÆĆ¾ŌŚĖ®ČÜŅŗÖŠĖ®½āÉś³ÉĒāŃõ»ÆĆ¾£»¶čŠŌµē¼«µē½āMgCl2ČÜŅŗŅõ¼«ĒāĄė×ӵƵ½µē×ÓÉś³ÉĒāĘų£¬Ė®µÄµēĄėĘ½ŗāĘĘ»µ£¬Ė®µēĄėÉś³ÉĒāŃõøłĄė×ÓÅضČŌö“ó£¬ŗĶĆ¾Ąė×ÓŠĪ³ÉĒāŃõ»ÆĆ¾³Įµķ£¬ŗĻ²¢Š“³öµē¼«·“Ó¦£»
£Ø3£©Č¼ĮĻµē³ŲÖŠÕż¼«ÉĻŹĒ¹żŃõ»ÆĒāµĆµ½µē×ÓÉś³ÉĖ®£»ŅĄ¾Żµē½āÖŹČÜŅŗPH±ä»Æ½įŗĻµē³Ų·“Ó¦¼ĘĖćĆ¾Ąė×ÓÅØ¶Č£»PH=6¼ĘĖćĒāŃõøłĄė×ÓÅØ¶Č£¬½įŗĻÉś³ÉĆ¾Ąė×ÓÅØ¶Č¼ĘĖćÅضČÉĢŗĶČܶȻż³£Źż±Č½Ļ·ÖĪöŹĒ·ńÉś³ÉĒāŃõ»ÆĆ¾³Įµķ£»
£Ø4£©Įņ“śĮņĖįÄĘČÜŅŗ³Ź¼īŠŌ£¬Ń”Ōń¼īŹ½µĪ¶Ø¹Ü£¬øł¾Ż·“Ó¦·½³ĢŹ½¼°µĪ¶ØŹż¾Ż¼ĘĖć³ö“ÖÅšÖŠÅšµÄŗ¬Į森
½ā“š ½ā£ŗ£Ø1£©ÓĆH2SO4µ÷pH2”«3£¬ÅšÉ°ÖŠµÄNa2B4O7ŌŚĖįČÜŅŗÖŠÉś³ÉH3BO3 £¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗB4O72-+2H++5H2O=4H3BO3£¬XĪŖH3BO3¾§Ģå¼ÓČČĶŃĖ®µÄ²śĪļÅŠ¶ĻĪŖB2O3£¬ÓėMg·“Ӧɜ³É“ÖÅšŗĶŃõ»ÆĆ¾£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ3Mg+B2O3$\frac{\underline{\;øßĪĀ\;}}{\;}$2B+3MgO£¬
¹Ź“š°øĪŖ£ŗB4O72-+2H++5H2O=4H3BO3£»3Mg+B2O3$\frac{\underline{\;øßĪĀ\;}}{\;}$2B+3MgO£»
£Ø2£©MgCl2•7H2OŠčŅŖŌŚHCl·ÕĪ§ÖŠ¼ÓČČ£¬ŹĒĪŖĮĖ·ĄÖ¹ĀČ»ÆĆ¾Ė®½āÉś³ÉĒāŃõ»ÆĆ¾£»ČōÓƶčŠŌµē¼«µē½āMgCl2ČÜŅŗ£¬Ņõ¼«ĒāĄė×ӵƵ½µē×ÓÉś³ÉĒāĘų£¬Ė®µÄµēĄėĘ½ŗāĘĘ»µ£¬Ė®µēĄėÉś³ÉĒāŃõøłĄė×ÓÅضČŌö“ó£¬ŗĶĆ¾Ąė×ÓŠĪ³ÉĒāŃõ»ÆĆ¾³Įµķ£¬ŗĻ²¢Š“³öŅõ¼«·“Ó¦Ź½ĪŖ£ŗ2H2O+Mg2++2e-=H2”ü+Mg£ØOH£©2”ż£»
¹Ź“š°øĪŖ£ŗ·ĄÖ¹MgCl2Ė®½āÉś³ÉMg£ØOH£©2£»2H2O+Mg2++2e-=H2”ü+Mg£ØOH£©2”ż£»
£Ø3£©Ć¾-H2O2ĖįŠŌČ¼ĮĻµē³ŲµÄ·“Ó¦»śĄķĪŖMg+H2O2+2H+ØTMg2++2H2O£¬Õż¼«ÉĻŹĒ¹żŃõ»ÆĒāµĆµ½µē×ÓÉś³ÉĖ®µÄ·“Ó¦£¬Õż¼«·“Ó¦Ź½H2O2+2H++2e-=2H2O£»ČōĘšŹ¼µē½āÖŹČÜŅŗpH=1£¬ŌņpH=2Ź±ČÜŅŗÖŠ£¬ĒāĄė×ÓÅØ¶Č¼õŠ”0.1mol/L-0.01mol/L=0.09mol/L£¬ŅĄ¾Ż·“Ó¦·½³ĢŹ½µĆµ½Mg2+Ąė×ÓÅضČ=0.045mol/L£»Ksp[Mg£ØOH£©2]=5.6”Į10-12£¬µ±ČÜŅŗpH=6Ź±£¬c£ØOH-£©=10-8mol/L£¬ŌņQc=c£ØMg2+£©”Įc2£ØOH-£©=0.045mol/L”Į10-16mol/L=4.5”Į10-18£¼Ksp[Mg£ØOH£©2]£¬ĖµĆ÷ĪŽĒāŃõ»ÆĆ¾³ĮµķÉś³É£»
¹Ź“š°øĪŖ£ŗH2O2+2H++2e-=2H2O£»0.045 mol•L-1£»Ć»ÓŠ£»
£Ø4£©Įņ“śĮņĖįÄĘČÜŅŗ³Ź¼īŠŌ£¬Ń”Ōń¼īŹ½µĪ¶Ø¹Ü£»Įņ“śĮņĖįÄʵÄĪļÖŹµÄĮæĪŖ£ŗ0.30mol/L”Į0.018L=0.0054mol£¬øł¾Ż¹ŲĻµŹ½£ŗB”«BI3”«$\frac{3}{2}$I2”«3S2O32-£¬n£ØB£©=$\frac{1}{3}$n£ØS2O32-£©=0.0018mol£¬
ÅšµÄÖŹĮæĪŖ£ŗ11g/mol”Į0.0018mol=0.0198g£¬“ÖÅšÖŠÅšµÄŗ¬ĮæĪŖ£ŗ$\frac{0.0198g}{0.02g}$”Į100%=99%£¬
¹Ź“š°øĪŖ£ŗ¼īŹ½£»99%£®
µćĘĄ ±¾Ģāæ¼²éĮĖŃĪĄąµÄĖ®½ā”¢Ōµē³ŲŌĄķŗĶµē½ā³ŲŌĄķµÄ·ÖĪö£¬³ĮµķČܽāĘ½ŗāµÄ¼ĘĖćÓ¦ÓĆ£¬ĢāÄæÄѶČÉŌ“󣬼ĘĖćŹĒÄŃµć£¬¼ĘĖ揱ŅŖ³ä·ÖĄūÓĆĢāÄæĖłøųŹżĮæ¹ŲĻµŗĶ±ķÖŠŹż¾Ż£¬øł¾ŻÖŹĮæ¹ŲĻµŗĶÖŹĮæ·ÖŹżµÄøÅÄīæģĖŁ½āĢā£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀĢÉ«Ö²Īļ½ųŠŠ¹āŗĻ×÷ÓĆŹ±£¬½«Ģ«ŃōÄÜ×Ŗ»ÆĪŖ»ÆѧÄÜ”°“¢“ꔱʚĄ“ | |
| B£® | ĪļÖŹ·¢Éś»Æѧ·“Ó¦¹ż³ĢÖŠŅ»¶Ø°éĖę×ÅÄÜĮæ±ä»Æ | |
| C£® | æɽ«·“Ó¦”°NaOH+HClØTNaCl+H2O”±µÄ»ÆѧÄÜĶعżŌµē³Ų×Ŗ»ÆĪŖµēÄÜ | |
| D£® | »Æѧ·“Ó¦Ź¹·ÅČČ»¹ŹĒĪüČČ£¬Č”¾öÓŚÉś³ÉĪļ¾ßÓŠµÄ×ÜÄÜĮæŗĶ·“Ó¦Īļ¾ßÓŠµÄ×ÜÄÜĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | KNO3ČÜŅŗ | B£® | FeCl3ČÜŅŗ | C£® | Al2£ØSO4£©3ČÜŅŗ | D£® | FeSO4ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹŌ¹Ü”¢Õō·¢Ćó | B£® | ŹŌ¹Ü”¢ÉÕ± | C£® | ŹŌ¹Ü”¢Ę½µ×ÉÕĘæ | D£® | Õō·¢Ćó”¢Ō²µ×ÉÕĘæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
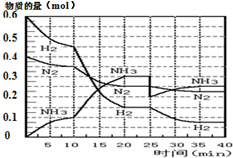 °±µÄŗĻ³ÉŌĄķĪŖ£ŗN2£Øg£©+3H2£Øg£©?2NH3£Øg£©£»”÷H=-92.4KJ•mol-1£®ĻÖŌŚ500”ę”¢20MPaŹ±£¬½«N2”¢H2ÖĆÓŚŅ»øöČŻ»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ·¢Éś·“Ó¦£¬·“Ó¦¹ż³ĢÖŠø÷ĪļÖŹµÄĪļÖŹµÄĮæ±ä»ÆČēĶ¼£®
°±µÄŗĻ³ÉŌĄķĪŖ£ŗN2£Øg£©+3H2£Øg£©?2NH3£Øg£©£»”÷H=-92.4KJ•mol-1£®ĻÖŌŚ500”ę”¢20MPaŹ±£¬½«N2”¢H2ÖĆÓŚŅ»øöČŻ»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ·¢Éś·“Ó¦£¬·“Ó¦¹ż³ĢÖŠø÷ĪļÖŹµÄĪļÖŹµÄĮæ±ä»ÆČēĶ¼£®| T/K | 303 | 313 | 323 |
| NH3Éś³ÉĮæ/£Ø10-6mol£© | 4.8 | 5.9 | 6.0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆŹ³“×ĒåĻ“ČČĖ®ĘæÖŠµÄĖ®¹ø£»ÓĆĆ×ĢĄ¼ģŃéµāŃĪÖŠŗ¬ÓŠµāĖį¼Ų | |
| B£® | Š”ĖÕ“ņæÉÖŠŗĶĪøĖį£¬ČČ“æ¼īČÜŅŗæɳżÓĶĪŪ£»Š”ĖÕ“ņŗĶ“æ¼īæÉŅŌÓĆŹÆ»ŅĖ®¼ų±š | |
| C£® | Óƶ”“ļ¶ūŠ§Ó¦Ēų±š¼¦µ°°×ČÜŅŗŗĶŹ³ŃĪĖ® | |
| D£® | ”°84Ļū¶¾Ņŗ”±ŗĶ½ą²Ž¼ĮÄÜĶ¬Ź±Ź¹ÓĆ£¬³żĪŪ¹øŠ§¹ūøüŗĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ČēĶ¼ŹĒæÉÄę·“Ó¦X2+3Y2?2Z2 ŌŚ·“Ó¦¹ż³ĢÖŠµÄ·“Ó¦ĖŁĀŹ£ØV£©ÓėŹ±¼ä£Øt£©µÄ¹ŲĻµĒśĻߣ¬ĻĀĮŠŠšŹö²»ÕżČ·µÄŹĒ£Ø””””£©
ČēĶ¼ŹĒæÉÄę·“Ó¦X2+3Y2?2Z2 ŌŚ·“Ó¦¹ż³ĢÖŠµÄ·“Ó¦ĖŁĀŹ£ØV£©ÓėŹ±¼ä£Øt£©µÄ¹ŲĻµĒśĻߣ¬ĻĀĮŠŠšŹö²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | t2Ź±£¬·“Ó¦µ½“ļĻŽ¶Č | B£® | t2-t3£¬·“Ó¦ČŌŌŚ½ųŠŠ | ||
| C£® | t1Ź±£¬Ö»ÓŠÕż·½Ļņ·“Ó¦ | D£® | t2-t3£¬ø÷ĪļÖŹµÄÅØ¶Č²»ŌŁ·¢Éś±ä»Æ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com