
��ѧʵ��װ�õ���ȷ������ʵ��ɰܵĹؼ�����ͼ����ѧ��ѧ�г�����ʵ��װ�á�

(1)������װ���Ʊ����壺
����B��ʢ��Ũ���ᣬ��A��B��C��Ϻ�����ȡ��������________��
a��H2 b��H2S
c��CO2  d��C2H4
d��C2H4
�� Ҫ������ȡ���õ������NH3����ȷ�����������________(������װ��˳���������ı����ĸ)��������ѡ�õĹ���ҩƷ��________________��
Ҫ������ȡ���õ������NH3����ȷ�����������________(������װ��˳���������ı����ĸ)��������ѡ�õĹ���ҩƷ��________________��
������H2O2��MnO2����ȡ���ռ������O2����Ӧѡ�����ȷ���������________(������װ��˳���������ı����ĸ)�����������ռ����ķ�����__________________________
_____________________________________��
(2)��ͬѧ��A��B�������֤���ᡢ̼�ᡢ�����������ǿ��ʱ��������Ӧװ��____________(����������)�У�Bװ���з�����Ӧ�����ӷ���ʽΪ_________________
______________________________________________________________��
������(1)��B�е�Ũ������Ժ����ⷴӦ��Cװ��ֻ���ռ��ܶȱȿ���������壬��������ϩ�����ϣ�����ֻ��ѡc�������ù����������ƺ�Ũ��ˮ���Կ�����ȡ�����������ܶȱȿ���С�����Բ��������ſ������ռ������������ܶȱȿ��������Բ��������ſ������ռ�����ȷ�����ΪABC������O2�ռ����ķ����ǽ������ǵ�ľ����������ƿ�ڣ���ľ����ȼ����˵�������ռ����ˡ�
(2)������A��B�����֤���ᡢ̼�ᡢ�����������ǿ��ʱ�����Բ���ǿ��������ķ�����A�����������̼���Ʒ�Ӧ��ȡ������̼����������̼ͨ��B�еĴ�������У��ɷ�Ӧ���ɴ������CaCO3������
�𰸡�(1)��c����AFE�������������ơ���ABC���������ǵ�ľ����������ƿ�ڣ���ľ����ȼ����˵�������ռ����ˡ�(2)��Һ©���� Ca2����2ClO����CO2��H2O===CaCO3����2HClO
Ca2����2ClO����CO2��H2O===CaCO3����2HClO


 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾװ���ռ�����8�����壨ͼ����ƿ��λ�ò��ñ仯����
 ��H2����Cl2����CH4����HCl����NH3����NO����H2S����SO2
��H2����Cl2����CH4����HCl����NH3����NO����H2S����SO2
����1������ƿ�Ǹ���ģ�����B�ڽ����ռ���������________��д��ţ���
��2������ƿ����ˮ�����ռ���������________����ʱ������________���롣
����3������ƿ�Ǹ���ģ�����A�ڽ��������ռ���������________��
����4��������ƿ��װ��Ũ����ʹ����������ô�װ���������������_____����ʱ������____�ڽ��롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Һ����Һ�ı�����(����)
A���ж����ЧӦ
B����ɢ�����ӵ�ֱ����С
C������ͨ����ֽ
D����ɢ�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҴӺ���������ȡ��IJ��������У�����ѡ�ò���ȷ����
A����ȡ3 g���ҵĸɺ�������������ƽ
B�����ոɺ�������ȫ��ɻҽ�����������
C��������к�ĺ����Һ�ˮ�Ļ�����©��
D�������Ȼ�̼��������ĺ����ҽ�ȡҺ����ȡ�⡪����Һ©��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ֽ��������������һ����Ҫ��ʵ�鷽������ͼ��ʾ��ʵ��(�ɼ���)�У�������ֽ��ѡ�á�����Ӧ���۶���ȷ��һ����
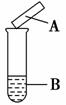
| ѡ�� | �Լ�B | ʪ�����ֽA | ���� | ���� |
| A | ��ˮ | ������ֽ | ���� | ����������� |
| B | Ũ��ˮ����ʯ�� | ��ɫʯ����ֽ | ��� | ����Ϊ�������� |
| C | Na2SO3������ | Ʒ����ֽ | ��ɫ | SO2����Ư���� |
| D | Cu��Ũ���� | KI������ֽ | ���� | NO2Ϊ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ѧʵ���еĺܶ�����������������ȡ�ģ���͵�������ȡ����������������HCl���ʣ�Ҫ��ȥHCl���ʶ��õ�������Ŀ�����壬����ͼʾװ�á�������ƿʢװ���DZ���NaHCO3��Һ�����������������������ij���װ��
A��H2������������������ B��Cl2
C��SO2 D��CO2

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӻ�������ȡ���ʵ������У��漰���в�����������ȷ����

A�����������ճɻҡ����� B�����˵ú�I������Һ
C���ų���ı���Һ D������Ⲣ���ձ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������淋Ļ�ѧʽΪ(NH4)2SO4��FeSO4��6H2O������Ī���Σ��Ƿ�����ѧ�г����Ļ�ԭ����ij��ѧ�о�С���������ʵ�����Ʊ�Ī���β��ⶨ��������淋Ĵ��ȡ�
����һ����м�Ĵ������������ʢ��������м����ƿ�м���Na2CO3��Һ�����ȡ����ˡ�ϴ�ӡ����������������Ϊm1��
�������FeSO4���Ʊ�����������м���뵽һ������ϡ�����У���ַ�Ӧ����˲���������ˮϴ����ƿ����ֽ����Һ��ϴ��Һ��ȫת�����������С������������أ�������Ϊm2��
����������������淋��Ʊ���ȷ��ȡ����������(NH4)2SO4���롰��������е��������У���������һ��ʱ���ֹͣ����ȴ������������什ᾧ����ˡ���������ˮ�Ҵ�ϴ�Ӳ���Ȼ����������þ���������
�����ģ��ñ�ɫ���ⶨ��������淋Ĵ��ȡ�
�ش��������⣺
(1)�������г�ȡ��(NH4)2SO4����Ϊ__________________________________________��
(2)����м��Na2CO3��Һ������Ŀ����_________________________________________________��

�Ʊ�FeSO4��Һʱ������ͼװ�ó��ȹ��ˣ�ԭ����_________________________________________________��
�ڽ�(NH4)2SO4��FeSO4��Ϻ���ȡ�Ũ����ֹͣ���ȵ�ʱ����_____________________________________��
�۱�ɫ���ⶨ��������林��ȵ�ʵ�鲽��Ϊ��Fe3����ɫ�����ơ����������������Һ�����ơ���ɫ�ⶨ����ɫ�ʹ���Һ����ʱ�������������ϡ�����⣬��Ӧע���������____________________________________________��
�ܸ�ʵ������ͨ��______________________________
ȷ����������鱗�Ʒ�ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����þ��ҽҩ����������ҵӦ�ù㷺������þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3)Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�

(1)MgCO3��ϡ���ᷴӦ�����ӷ���ʽΪ ��
(2)����H2O2����ʱ��������Ӧ�Ļ�ѧ����ʽΪ ��
(3)����2�ijɷ��� (�ѧʽ)��
 (4)���չ��̴������·�Ӧ��
(4)���չ��̴������·�Ӧ��
2MgSO4��C 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��
MgSO4��C MgO��SO2����CO��
MgO��SO2����CO��
MgSO4��3C MgO��S����3CO��
MgO��S����3CO��
������ͼװ�ö����ղ�����������зֲ����ջ��ռ���
��D���ռ������������ (�ѧʽ)��
��B��ʢ�ŵ���Һ������ (����ĸ)��
a.NaOH ��Һ b.Na2CO3��Һ c.ϡ���� d.KMnO4��Һ
��A�еõ��ĵ���ɫ�������ȵ�NaOH��Һ��Ӧ��������Ԫ�����̬Ϊ��4��д���÷�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com