
�����ͼ��ѡ�ñ�Ҫ��װ�ý��е�ⱥ��ʳ��ˮ��ʵ�飬Ҫ��ⶨ�������������(����25 mL)�������������������ԡ�
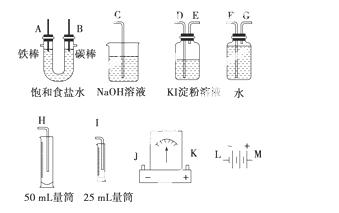
(1)A�������ĵ缫��Ӧʽ��________________________________________________��
B�������ĵ缫��Ӧʽ��__________________________________________________��
��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ______________________________________________��
(2)��Դ����������A��B��������ȷ����˳��Ϊ��L��(����)��(����)��(����)��(����)��M��
(3)�����������ʵ��װ��ʱ�����ӿڵ���ȷ����˳��Ϊ��________��________��________��A��B��________��________��________��
(4)��ʵ���У�ʢ ��KI������Һ�������з�����Ӧ�����ӷ���ʽΪ__________________________________________________��
��KI������Һ�������з�����Ӧ�����ӷ���ʽΪ__________________________________________________��
(5)��֪����ʳ��ˮ50 mL��ijʱ�̲��H2���Ϊ5.6 m L(��״��)����ʱ��ҺpHԼΪ__________________________________________________��
L(��״��)����ʱ��ҺpHԼΪ__________________________________________________��
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ��W��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ34��Mԭ�ӵ����������������������֮��Ϊ34����Mԭ�ӵ���������Yԭ�ӵ�2����XԪ�ص��������������۾���ֵ֮��Ϊ2��N����Z����W���İ뾶��С��������WN������Ϊ���壬�ݴ˻ش��������⣺
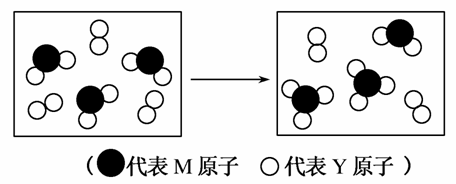
(1)W��Y�ɷֱ��γ�10���Ӻ�18���ӵķ��ӣ�д����18���ӷ���ת����10���ӷ��ӵĻ�ѧ����ʽ��
___________________________________________________________��
(2)��ͼ��ʾ����������Ԫ����ɵ����������һ�������µ��ܱ������г�ַ�Ӧǰ���ת����ϵ����д����ת�����̵Ļ�ѧ����ʽ��_________��
(3)A��B��Ϊ����������Ԫ���е�����Ԫ����ɵ�ǿ����ʣ������Ԫ�ص�ԭ�Ӹ���֮�Ⱦ�Ϊ1��1��1�����ڸ��Ե�ˮ��Һ�У�A������ˮ�ĵ��룬B�ܴٽ�ˮ�ĵ��룬��A�Ļ�ѧʽΪ________��B�ĵ���ʽ��________��
(4)���ʢ������XY2���ܱ�����(�����ƶ��Ļ���)����ѹ��������������������_________________________________________��ԭ����______
_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����д�йصĵ缫��Ӧʽ���ܷ���ʽ��
(1)�ö��Ե缫���AgNO3��Һ��
������Ӧʽ_____________________________________________________��
������Ӧʽ_____________________________________________________��
�ܷ�Ӧ���ӷ���ʽ________________________________________________��
(2)�ö��Ե缫���MgCl2��Һ
������Ӧʽ______________________________________________________��
������Ӧʽ______________________________________________________��
�ܷ�Ӧ���ӷ���ʽ________________________________________________��
(3)�������缫���NaCl��Һ
������Ӧʽ______________________________________________________��
������Ӧʽ______________________________________________________��
�ܻ�ѧ����ʽ____________________________________________________��
(4)��ͭ���缫���������Һ
������Ӧʽ_____________________________________________________��
������Ӧʽ_____________________________________________________��
�ܷ�Ӧ���ӷ���ʽ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ת������Դ���úͻ�����������Ҫ�о����⡣�������������ж��ַ�����
(1)���ռ�����H2S�����Һ���뵽��ͼ��ʾ�ĵ��ص����������е�⡣���������������������·�Ӧ��
S2����2e��===S��(n��1)S��S2��===S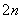
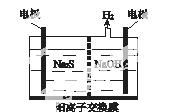
��д�����ʱ�����ĵ缫��Ӧʽ��________________��
�ڵ�������������Һ��ϡ�����ữ�õ����ʣ������ӷ���ʽ��д��__________________________��
(2)��H2S�Ϳ����Ļ������ͨ��FeCl3��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת����ͼ��ʾ��
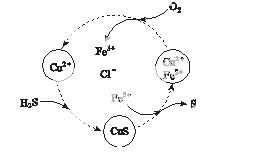
����ͼʾ ��ת���У����ϼ�
��ת���У����ϼ� �����Ԫ����________��
�����Ԫ����________��
�ڷ�Ӧ�е���1 m ol H2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬������O2�����ʵ���Ϊ________��
ol H2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬������O2�����ʵ���Ϊ________��
�����¶�һ���Ͳ�������Һ�������£�����ͨ�������壬����ֽ��衣��ʹ���ɵ������в���CuS���ɲ�ȡ�Ĵ�ʩ��________________��
(3)H2S�ڸ����·ֽ�������������H2������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ����������ͼ��ʾ��H2S�ڸ����·ֽⷴӦ�Ļ�ѧ����ʽΪ__________________________________��
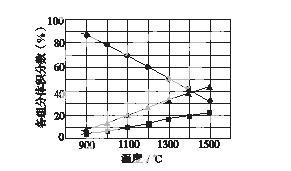
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������ڳ��ͷŵ�ʱ�����ķ�ӦΪ��
Fe��NiO2��2H2O Fe(OH)2��Ni(OH)2������˵������ȷ����(����)
Fe(OH)2��Ni(OH)2������˵������ȷ����(����)
A���ŵ�ʱ�������Ϸ�����Ӧ��������Fe
B���ŵ�ʱ��������Ӧ�ǣ�NiO2��2e����2H��===Ni(OH)2
C�����ʱ��������Ӧ�ǣ�N i(OH)2��2e����2OH��===NiO2��2H2O
i(OH)2��2e����2OH��===NiO2��2H2O
D�����ʱ����������pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ͼ���Ӧ���ϵ��ǣ� ��
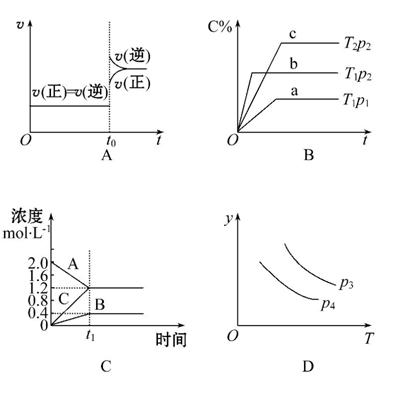
A.���ڴﵽƽ��״̬��N2(g)+3H2(g) 2NH3(g)��t0ʱ�̳�����һ����NH3��ƽ�������ƶ�
2NH3(g)��t0ʱ�̳�����һ����NH3��ƽ�������ƶ�
B.p2>p1��T1>T2
C.��ͼ���ʾ�ķ���ʽΪ��2A====B+3C
D.���ڷ�Ӧ2X(g)+3Y(g)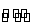 2Z(g)����H<0��y���Ա�ʾY�İٷֺ���
2Z(g)����H<0��y���Ա�ʾY�İٷֺ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���о��л�����ʱ������16 8O��Ϊʾ��ԭ�ӡ�16 8O��ԭ�Ӻ�����������
A��8 B��18 C��10 D��28
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���淴ӦCO(g)+H2O(g) CO2(g)+H2(g),�ﵽƽ����йػ�ѧ��Ӧ�ȵ�˵����ȷ����
A��V��=0 B��V��=0 C��V��= V���0 D��V��= V��=0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����
A��ij���ռ乹��Ϊ�����Σ������һ���Ǽ��Է���
B��ij���ռ乹��ΪV�Σ�������ԭ��һ���йµ��Ӷ�
C��sp3�ӻ��������ͬһ��ԭ�������������s�����p�����������γɵ�һ���¹��
D����������ԭ�Ӳ�ȡsp3�ӻ�����ɼ��ķ��������幹�Ͷ�����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com