
”¾ĢāÄæ”æ äåŅŅĶéŌŚ²»Ķ¬ČܼĮÖŠÓė NaOH ·¢Éś²»Ķ¬ĄąŠĶµÄ·“Ó¦£¬Éś³É²»Ķ¬µÄ·“Ó¦²śĪļ”£Ä³ Ķ¬Ń§ŅĄ¾ŻäåŅŅĶéµÄŠŌÖŹ£¬ÓĆÓŅĶ¼ŹµŃé×°ÖĆ £ØĢś¼ÜĢØ”¢¾Ę¾«µĘĀŌ£© Ńé֤Ȕ“ś·“Ó¦ŗĶĻūČ„ ·“Ó¦µÄ²śĪļ£¬ĒėÄćŅ»Ęš²ĪÓėĢ½¾æ”£

ŹµŃé²Ł×÷¢ń£ŗŌŚŹŌ¹ÜÖŠ¼ÓČė 5mL1mol/LNaOH ČÜŅŗŗĶ 5mL äåŅŅĶ飬Õńµ“”£ ŹµŃé²Ł×÷ II£ŗ½«ŹŌ¹ÜČēĶ¼¹Ģ¶Øŗó£¬Ė®Ō”¼ÓČČ”£
£Ø1£© ÓĆĖ®Ō”¼ÓČȶų²»Ö±½ÓÓĆ¾Ę¾«µĘ¼ÓČȵÄŌŅņŹĒ ”£
£Ø2£© ¹Ū²ģµ½ ĻÖĻóŹ±£¬±ķĆ÷äåŅŅĶéÓė NaOH ČÜŅŗŅŃĶźČ« ·“Ó¦”£
£Ø3£© ¼ų¶ØÉś³ÉĪļÖŠŅŅ“¼µÄ½į¹¹£¬æÉÓĆµÄ²ØĘ׏Ē ”£
£Ø4£© ĪŖÖ¤Ć÷äåŅŅĶéŌŚ NaOH ŅŅ“¼ČÜŅŗÖŠ·¢ÉśµÄŹĒĻūČ„·“Ó¦£¬ŌŚÄćÉč¼ĘµÄŹµŃé·½°øÖŠ£¬ ŠčŅŖ¼ģŃéµÄŹĒ £¬¼ģŃéµÄ·½·ØŹĒ ”£
£ØŠčĖµĆ÷£ŗĖłÓƵďŌ¼Į”¢¼ņµ„µÄŹµŃé²Ł×÷¼°Ō¤²ā²śÉśµÄŹµŃéĻÖĻ󣩔£
”¾“š°ø”æ(9·Ö)
£Ø1£©äåŅŅĶé·ŠµćµĶ£¬¼õÉŁäåŅŅĶéµÄĖšŹ§£Ø2·Ö£©
£Ø2£©ŹŌ¹ÜÄŚČÜŅŗ¾²ÖĆŗó²»·Ö²ć£Ø2·Ö£©
£Ø3£©ŗģĶā¹āĘ×”¢ŗĖ“Ź²ÕńĒāĘ×£Ø2·Ö£©
£Ø4£©Éś³ÉµÄĘųĢå£Ø1·Ö£©½«Éś³ÉµÄĘųĢåĻČĶعżŹ¢ÓŠĖ®µÄŹŌ¹Ü£¬ŌŁĶØČėŹ¢ÓŠKMnO4ČÜŅŗµÄŹŌ¹Ü£¬KMnO4ČÜŅŗĶŹÉ«(»ņÖ±½ÓĶØČėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ)£Ø2·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©äåŅŅĶé·ŠµćµĶ£¬äåŅŅĶéŅ×»Ó·¢£¬ÓĆĖ®Ō”¼ÓČČČČ¾łŌČ£¬¼õÉŁäåŅŅĶéµÄĖšŹ§£¬¹Ź“š°øĪŖ£ŗŹ¹ŹŌ¹ÜŹÜČČ¾łŌČ£¬¼õÉŁäåŅŅĶéµÄĖšŹ§£»
£Ø2£©äåŅŅĶé²»ČÜÓŚĖ®£¬æŖŹ¼ČÜŅŗ·Ö²ć£¬Éś³ÉµÄ²śĪļŅŅ“¼”¢äå»ÆÄʶ¼Ņ×ČÜÓŚĖ®£¬µ±ČÜŅŗ·Ö²ćĻūŹ§£¬±ķĆ÷äåŅŅĶéÓėNaOHČÜŅŗŅŃĶźČ«·“Ó¦£¬·“Ó¦»Æѧ·½³ĢŹ½ĪŖ£ŗCH3CH2Br+NaOH”śCH3CH2OH+NaBr£¬
¹Ź“š°øĪŖ£ŗŹŌ¹ÜÄŚČÜŅŗ¾²ÖĆŗó²»·Ö²ć£»
£Ø3£©ŅŅ“¼µÄµē×ÓŹ½£ŗ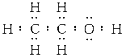 £¬½į¹¹Ź½ŹĒ£ŗ
£¬½į¹¹Ź½ŹĒ£ŗ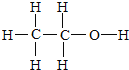 £¬ŅŅ“¼·Ö×Ó½į¹¹ÖŠÓŠČżÖÖĒāŌ×Ó£¬ĖüĆĒµÄ±ČĪŖ3£ŗ2£ŗ1£¬ĄūÓĆŗĖ“Ź²ÕńĒāĘ×æɼģ²ā£¬Ņ²æÉÓĆŗģĶā¹āĘ×¼ģ²ā£¬
£¬ŅŅ“¼·Ö×Ó½į¹¹ÖŠÓŠČżÖÖĒāŌ×Ó£¬ĖüĆĒµÄ±ČĪŖ3£ŗ2£ŗ1£¬ĄūÓĆŗĖ“Ź²ÕńĒāĘ×æɼģ²ā£¬Ņ²æÉÓĆŗģĶā¹āĘ×¼ģ²ā£¬
¹Ź“š°øĪŖ£ŗŗģĶā¹āĘ×”¢ŗĖ“Ź²ÕńĒāĘ×£»
£Ø4£©äåŅŅĶéŌŚĒāŃõ»ÆÄʵē¼ČÜŅŗÖŠ·¢ÉśĻūČ„·“Ó¦£¬·“Ó¦¶Ļ1ŗÅCµÄC-Br¼ü£¬CH3CH2Br+NaOH”ś
CH2=CH2”ü+NaBr+H2O£¬Éś³ÉŅŅĻ©£¬Ö»ÓŠÖ¤Ć÷ŅŅĻ©µÄ“ęŌŚ¼“æÉÖ¤Ć÷·¢ÉśµÄŹĒĻūČ„·“Ó¦£¬·½·ØŹĒ£ŗ½«Éś³ÉµÄĘųĢåĻČĶعżŹ¢ÓŠĖ®µÄŹŌ¹Ü£¬ŌŁĶØČėŹ¢ÓŠĖįŠŌKMnO4ČÜŅŗµÄŹŌ¹Ü£¬5CH2=CH2+12KMnO4+18H2O”ś12MnSO4+6K2SO4+10CO2”ü+28H2O£¬ĖįŠŌKMnO4ČÜŅŗĶŹÉ«£¬£Ø»ņÖ±½ÓĶØČėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ£¬ŅŅĻ©Óėäåµ„ÖŹ·¢Éś¼Ó³É£ŗBr2+CH2=CH2”śBrCH2-CH2Br£¬ĖÄĀČ»ÆĢ¼ĶŹÉ«£©£¬
¹Ź“š°øĪŖ£ŗÉś³ÉµÄĘųĢ壻½«Éś³ÉµÄĘųĢåĻČĶعżŹ¢ÓŠĖ®µÄŹŌ¹Ü£¬ŌŁĶØČėŹ¢ÓŠĖįŠŌKMnO4ČÜŅŗµÄŹŌ¹Ü£¬ĖįŠŌKMnO4ČÜŅŗĶŹÉ«£Ø»ņÖ±½ÓĶØČėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ£¬ĖÄĀČ»ÆĢ¼ĶŹÉ«£©£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¼×”¢ŅŅ”¢±ūČżÖÖČÜŅŗÖŠŗ¬ÓŠŅ»ÖÖĀ±ĖŲĄė×Ó(Cl-”¢Br-”¢I-)£¬Ļņ¼×ÖŠ¼ÓČėµķ·ŪČÜŅŗŗĶĀČĖ®£¬ŌņČÜŅŗ±äĪŖ³ČÉ«£¬ŌŁ¼Ó±ūČÜŅŗ£¬ŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ£¬Ōņ¼×”¢ŅŅ”¢±ūÖŠŅĄ“Īŗ¬ÓŠ£Ø £©
A. Br-ӢI-ӢCl-B. Br-ӢCl-ӢI-C. I-ӢBr-ӢCl-D. Cl-ӢI-ӢBr-
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ(1)ĻąĶ¬ĪĀ¶ČŗĶŃ¹ĒæĻĀ£¬µČÖŹĮæµÄSO2ŗĶO2”£¶žÕßĢå»ż±ČĪŖ________£¬·Ö×ÓøöŹż±ČĪŖ________£¬ĆܶȱČĪŖ________”£
(2)ŌŚ±ź×¼×“æöĻĀ£¬ÓÉCOŗĶCO2×é³ÉµÄ»ģŗĻĘųĢåĪŖ6.72 L£¬ÖŹĮæĪŖ12 g£¬“Ė»ģŗĻĪļÖŠCOŗĶCO2ĪļÖŹµÄĮæÖ®±ČŹĒ________£¬COµÄĢå»ż·ÖŹżŹĒ________£¬COµÄÖŹĮæ·ÖŹżŹĒ________£¬CŗĶOŌ×ÓøöŹż±ČŹĒ________£¬»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæŹĒ________£¬ĆܶȏĒ________g”¤L1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ²ÄĮĻÓėČĖĄąÉś»īĆÜĒŠĻą¹Ų£¬ĻĀĮŠĪļĘ·ÖŠ×īÄŃ½µ½āµÄŹĒ
A. ÕęĖæĪ§½ķ B. ŠūÖ½ C. PVCĖÜĮĻæØʬ D. µķ·Ū×öµÄ²Ķ¾ß
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©Ēėøł¾Ż¹ŁÄÜĶŵIJ»Ķ¬¶ŌĻĀĮŠÓŠ»śĪļ½ųŠŠ·ÖĄą£ŗ
¢Ł(CH3)3CCH2OH ¢Ś ¢Ū
¢Ū![]()
¢Ü![]() ¢Ż
¢Ż![]() ¢Ž
¢Ž![]()
ÉĻŹöĪļÖŹÖŠ£¬ŹōÓŚ·¼Ļć“¼µÄŹĒ_________£¬ŹōÓŚ·ÓĄąµÄŹĒ___________
ŹōÓŚōČĖįĄąµÄŹĒ__________£¬ŹōÓŚČ©ĄąµÄŹĒ__________ĢīŠņŗÅ
£Ø2£©ĻĀĮŠĪļÖŹÖŠ»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄÓŠ__________£¬»„ĪŖĶ¬ĖŲŅģŠĪĢåµÄÓŠ______£¬
»„ĪŖĶ¬Ī»ĖŲµÄÓŠ_____________£¬ŹĒĶ¬Ņ»ÖÖĪļÖŹµÄÓŠ________(ĢīŠņŗÅ)”£

(3)°“ŅŖĒ󊓳öĻĀĮŠ»Æѧ·½³ĢŹ½”£
¢Ł ¼×±½”śTNT ___________________________£»
¢Ś 2-äå±ūĶ锜±ūĻ©____________________________£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ(1)ĻÖÓŠĢ¼”¢¶žŃõ»ÆĢ¼”¢Ńõ»ÆĢś”¢ŹÆ»ŅĖ®”¢Ļ”ĮņĖįŗĶ“æ¼īČÜŅŗµČ6ÖÖĪļÖŹ”£

¢Ł³żĻ”ĮņĖįĶā£¬ŹŌ·Ö±š½«ĘäĖūĪļÖŹµÄ»ÆѧŹ½ĢīČėČēĶ¼ĻąÓ¦µÄ5øöŌ²Č¦ÄŚ£¬Ź¹ĆæøöŌ²Č¦ÄŚµÄĪļÖŹ¶¼ÄÜÓėĻąĮŚĪļÖŹ·¢Éś·“Ó¦”£
¢ŚÓĆ»Æѧ·½³ĢŹ½½«ĖüĆĒÓėĻąĮŚĪļÖŹ¼ä·¢ÉśµÄ·“Ó¦±ķŹ¾³öĄ“£ŗ
_____________________________£»
_____________________________£»
_____________________________£»
_____________________________£»
_____________________________£»
_____________________________ӣ
(2)ŅŌĻĀ±ķŹ¾µÄŹĒĢ¼¼°Ęä»ÆŗĻĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£ŗ
C![]() CO2
CO2![]() H2CO3
H2CO3![]() CaCO3
CaCO3![]() CO2
CO2
ĘäÖŠÉę¼°µÄ»ł±¾·“Ó¦ĄąŠĶŅĄ“ĪĪŖ_________”¢__________”¢__________”¢__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅ»ÖÖŅŌ»ĘĶæóŗĶĮņ»ĒĪŖŌĮĻÖĘČ”ĶŗĶĘäĖū²śĪļµÄŠĀ¹¤ŅÕ£¬ŌĮĻµÄ×ŪŗĻĄūÓĆĀŹ½Ļøß”£ĘäÖ÷ŅŖĮ÷³ĢČēĻĀ£ŗ

ŅŃÖŖ£ŗ”°·“Ó¦¢ņ”±µÄĄė×Ó·½³ĢŹ½ĪŖCu2++CuS+4C1-==2[CuCl2]-+S
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĢśŗģµÄ»ÆѧŹ½ĪŖ__________________£»
£Ø2£©”°·“Ó¦¢ņ”±µÄ»¹Ō¼ĮŹĒ_______________(Ģī»ÆѧŹ½)£»
£Ø3£©”°·“Ó¦III”±µÄĄė×Ó·½³ĢŹ½ĪŖ____________________________________£»
£Ø4£©»ŌĶæóµÄÖ÷ŅŖ³É·ÖŹĒCu2S£¬æÉÓÉ»ĘĶæó(Ö÷ŅŖ³É·ÖCuFeS2)Ķعżµē»Æѧ·“Ó¦×Ŗ±ä¶ų³É£¬ÓŠ¹Ų×Ŗ»ÆČēĻĀČēĶ¼ ĖłŹ¾”£×Ŗ»ÆŹ±Õż¼«µÄµē¼«·“Ó¦Ź½ĪŖ___________________”£
£Ø5£©“Ó»ŌĶæóÖŠ½žČ”ĶŌŖĖŲ£¬æÉÓĆFeCl3×÷½žČ”¼Į”£
¢Ł·“Ó¦Cu2S£«4FeCl32CuCl2£«4FeCl2£«S£¬ĆæÉś³É1mol CuCl2£¬·“Ó¦ÖŠ×ŖŅʵē×ӵďżÄæĪŖ______£»½žČ”Ź±£¬ŌŚÓŠŃõ»·¾³ĻĀæÉĪ¬³ÖFe3+½ĻøßÅØ¶Č”£ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ__________”£
¢Ś½žČ”¹ż³ĢÖŠ¼ÓČėĻ“µÓ¼ĮČܽāĮņŹ±£¬ĶŌŖĖŲµÄ½žČ”ĀŹµÄ±ä»ÆČēĶ¼£¬ĘäŌŅņŹĒ_______________”£


£Ø6£©CuClŠü×ĒŅŗÖŠ¼ÓČėNa2S£¬·¢ÉśµÄ·“Ó¦ĪŖ2CuCl(s)£«S2£(aq)![]() Cu2S(s)£«2Cl£(aq)£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżK £½__________________[ŅŃÖŖKsp(CuCl)£½1.2”Į10£6£¬ Ksp(Cu2S)£½2.5”Į10£43]”£
Cu2S(s)£«2Cl£(aq)£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżK £½__________________[ŅŃÖŖKsp(CuCl)£½1.2”Į10£6£¬ Ksp(Cu2S)£½2.5”Į10£43]”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĪļÖŹ¶¼ÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«£¬µ«ŌĄķÓėĘäĖūĪļÖŹ²»Ķ¬µÄŹĒ£Ø £©
A. SO2 B. Na2O2 C. HClO D. ĘÆ°×·Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹŅĪĀĻĀ£¬ÓĆ0.100 mol”¤L-1NaOHČÜŅŗ·Ö±šµĪ¶Ø20.00 mL 0.100 mol”¤L-1µÄŃĪĖįŗĶ“×Ėį£¬µĪ¶ØĒśĻßČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ

A£®¢ņ±ķŹ¾µÄŹĒµĪ¶Ø“×ĖįµÄĒśĻß
B£®pH=7Ź±£¬µĪ¶Ø“×ĖįĻūŗÄV£ØNaOH)Š”ÓŚ20 mL
C£®V£ØNaOH)=20.00 mLŹ±£¬Į½·ŻČÜŅŗÖŠc£ØCl-)=c£ØCH3COO-)
D£®V£ØNaOH)=10.00 mLŹ±£¬“×ĖįČÜŅŗÖŠ:c£ØNa+)>c£ØCH3COO-)>c£ØH+)>c£ØOH-)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com