
·ÖĪö £Ø1£©øł¾Żn=$\frac{m}{M}$¼ĘĖć³ö¶žŃõ»ÆĢ¼µÄĪļÖŹµÄĮ棬ŌŁøł¾Żn=nNA¼ĘĖć³öŗ¬ÓŠŃõŌ×ÓŹżÄ攢øł¾ŻV=nVm¼ĘĖć³ö±źæöĻĀ¶žŃõ»ÆĢ¼µÄĢå»ż£»
£Ø2£©øł¾Żn=$\frac{m}{M}$¼ĘĖć³öĀČ»ÆĆ¾µÄĪļÖŹµÄĮ棬“Ó¶ųµĆ³öĆ¾Ąė×ÓµÄĪļÖŹµÄĮ棻øł¾Żn=nNA¼ĘĖć³öŗ¬ÓŠĄė×ÓŹżÄ棻øł¾ŻĀČ»ÆĆ¾µÄ»ÆѧŹ½¼ĘĖć³öŗ¬ÓŠĆ¾Ąė×ÓµÄĪļÖŹµÄĮ棬ŌŁøł¾Żm=nM¼ĘĖć³öÖŹĮ棻
£Ø3£©øł¾Żn=$\frac{N}{{N}_{A}}$¼ĘĖć³öļ§øłĄė×ÓµÄĪļÖŹµÄĮ棬øł¾Żm=nM¼ĘĖć³öļ§øłĄė×ÓµÄÖŹĮ棻ļ§øłĄė×ÓÖŠŗ¬ÓŠ11øöÖŹ×Ó”¢10øöµē×Ó£¬øł¾ŻĘäĪļÖŹµÄĮæ¼ĘĖć³öŗ¬ÓŠŌÓÖŹµÄĪļÖŹµÄĮ棬n=nNA¼ĘĖć³öŗ¬ÓŠµē×ÓŹżÄ棻
£Ø4£©øł¾Żm=nM¼ĘĖć³öČżŃõ»ÆĮņµÄÖŹĮ棬¼ĘĖć³ö¶žŃõ»ÆĮņµÄĪļÖŹµÄĮ棬øł¾ŻV=nVm¼ĘĖć³ö±źæöĻĀ¶žŃõ»ÆĮņµÄĢå»ż£»ĮņČ¼ÉÕÉś³É¶žŃõ»ÆĮņ£¬¼ĘĖć³öĮņŌ×ÓµÄĪļÖŹµÄĮ棬µĆ³öĻūŗÄŃõĘųµÄĪļÖŹµÄĮ棬ŌŁ¼ĘĖć³ö±źæöĻĀŃõĘųµÄĢå»ż£»
£Ø5£©ĻąĶ¬ÖŹĮæŹ±£¬ĪļÖŹµÄĮæÓėĦ¶ūÖŹĮæ³É·“±Č£»·Ö×ÓŹżÓėĪļÖŹµÄĮæ³ÉÕż±Č£»ĻąĶ¬Ģõ¼žĻĀĢå»żÖ®±ČµČÓŚĪļÖŹµÄĮæÖ®±Č£»
£Ø6£©ĻąĶ¬Ģõ¼žĻĀ£¬µČĢå»żµÄĘųĢå¾ßÓŠĻąĶ¬µÄĪļÖŹµÄĮ攢·Ö×ÓŹż£»øł¾Ż¼×ĶéŗĶ¶žŃõ»ÆĢ¼µÄ·Ö×Ó×é³É¼ĘĖć³öŗ¬ÓŠŌ×ÓŹżÖ®±Č£»øł¾Żm=nM¼ĘĖć³ö¶žÕßµÄÖŹĮæÖ®±Č£»µČĢå»żµÄ¼×ĶéŗĶ¶žŃõ»ÆĢ¼¾ßÓŠĻąĶ¬µÄĪļÖŹµÄĮ棬øł¾ŻM=$\frac{m}{n}$¼ĘĖć³öĘ½¾łÄ¦¶ūÖŹĮ森
½ā“š ½ā£ŗ£Ø1£©8.8æĖCO2µÄĪļÖŹµÄĮæĪŖ£ŗ$\frac{8.8g}{44g/mol}$=0.2mol£¬0.2mol¶žŃõ»ÆĢ¼·Ö×ÓÖŠŗ¬ÓŠOŌ×ӵďżÄæĪŖ£ŗ0.2NA£»±ź×¼×“æöĻĀ0.2molµÄĢå»żŹĒ£ŗ22.4L/mol”Į0.2mol=4.48L£¬
¹Ź“š°øĪŖ£ŗ0.2mol£»0.2NA£»4.48L£»
£Ø2£©19æĖMgCl2µÄĪļÖŹµÄĮæĪŖ£ŗ$\frac{19g}{95g/mol}$=0.2mol£¬0.2molĀČ»ÆĆ¾ÖŠŗ¬ÓŠ0.2molMg2+£»0.2molĀČ»ÆĆ¾ÖŠŗ¬ÓŠ0.2molĆ¾Ąė×Ó”¢0.4molĀČĄė×Ó£¬×ܹ²ŗ¬ÓŠ0.6molĄė×Ó£¬ŗ¬ÓŠĄė×ÓŹżÄæ0.6NA£»ŗ¬ÓŠCl-0.4molµÄMgCl2ÖŠMg2+µÄĪļÖŹµÄĮæĪŖ£ŗ0.4mol”Į$\frac{1}{2}$=0.2mol£¬0.2molĆ¾Ąė×ÓµÄÖŹĮæĪŖ£ŗ24g/mol”Į0.2mol=4.8g£¬
¹Ź“š°øĪŖ£ŗ0.2mol£»0.6NA£»4.8g£»
£Ø3£©3.01”Į1023øöNH4+µÄĪļÖŹµÄĮæĪŖ£ŗ$\frac{3.01”Į1{0}^{23}}{6.02”Į1{0}^{23}}$=0.5mol£¬ÖŹĮæĪŖ£ŗ18g/mol”Į0.5mol=9g£»0.5molļ§øłĄė×ÓÖŠŗ¬ÓŠÖŹ×ÓµÄĪļÖŹµÄĮæĪŖ£ŗ0.5mol”Į11=5.5mol£¬ŗ¬ÓŠµē×ӵďżÄæĪŖ£ŗ0.5”Į10”ĮNA=5NA£¬
¹Ź“š°øĪŖ£ŗ0.5mol£»9g£»5NA£»
£Ø4£©1.5mol SO3µÄÖŹĮæĪŖ£ŗ80g/mol”Į1.5mol=120g£»1.5molČżŃõ»ÆĮņÖŠŗ¬ÓŠŃõŌ×ÓµÄĪļÖŹµÄĮæĪŖ£ŗ1.5mol”Į3=4.5mol£¬ŗ¬ÓŠ4.5molŃõŌ×ÓŠčŅŖ¶žŃõ»ÆĮņµÄĪļÖŹµÄĮæĪŖ£ŗ$\frac{4.5mol}{2}$=2.25mol£¬±ź×¼×“æöĻĀ2.25molSO2µÄĢå»żĪŖ£ŗ22.4L/mol”Į2.25mol=50.4L£»16gµ„ÖŹĮņµÄĪļÖŹµÄĮæĪŖ£ŗ$\frac{16g}{32g/mol}$=0.5mol£¬0.5molSĶźČ«Č¼ÉÕÉś³É0.5mol¶žŃõ»ÆĮņŠčŅŖĻūŗÄ0.5molŃõĘų£¬±źæöĻĀ0.5molŃõĘųµÄĢå»żĪŖ£ŗ22.4L/mol”Į0.5mol=11.2L£¬
¹Ź“š°øĪŖ£ŗ120g£»50.4£»11.2£»
£Ø5£©ĻąĶ¬ÖŹĮæµÄĮ½ÖÖĘųĢåCl2”¢O2£¬øł¾Żøł¾Żn=$\frac{m}{M}$æÉÖŖ£¬ĖüĆĒµÄĪļÖŹµÄĮæ±ČÓėĦ¶ūÖŹĮæ³É·“±Č£¬Ōņ¶žÕßµÄĪļÖŹµÄĮæÖ®±ČĪŖ£ŗ32g/mol£ŗ71g/mol=32£ŗ71£»ĖüĆĒĖłŗ¬·Ö×ÓŹż=ĪļÖŹµÄĮæ³ÉÕż±Č=32£ŗ71£»ŌŚĻąĶ¬Ģõ¼žĻĀĖüĆĒµÄĢå»ż±Č=ĪļÖŹµÄĮæÖ®±Č=32£ŗ71£¬
¹Ź“š°øĪŖ£ŗ32£ŗ71£»32£ŗ71£»32£ŗ71£»
£Ø6£©ŌŚĶ¬ĪĀĶ¬Ń¹ĻĀ£¬Ķ¬Ģå»żµÄ¼×Ķé£ØCH4£©ŗĶ¶žŃõ»ÆĢ¼¾ßÓŠĻąĶ¬µÄĪļÖŹµÄĮ棬¶žÕߵķÖ×ÓŹżĻąµČ£¬Ōņ·Ö×ÓŹżÖ®±ČĪŖ1£ŗ1£»ĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ1£¬Ō×Ó×ÜŹżÖ®±ČĪŖ£Ø1”Į4£©£ŗ£Ø1”Į3£©=4£ŗ3£»¶žÕßµÄĪļÖŹµÄĮæĻąµČ£¬ŌņÖŹĮæÖ®±ČÓėĦ¶ūÖŹĮæ³ÉÕż±Č£¬Ōņ¶žÕßµÄÖŹĮæÖ®±ČĪŖ£ŗ16g/mol£ŗ44g/mol=4£ŗ11£»µČĢå»żµÄ¼×Ķé£ØCH4£©ŗĶ¶žŃõ»ÆĢ¼¾ßÓŠĻąĶ¬µÄĪļÖŹµÄĮ棬Ōņ»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæĪŖ£ŗ$\frac{16g/mol+44g/mol}{1+1}$=30g/mol£¬
¹Ź“š°øĪŖ£ŗ1£ŗ1£»1£ŗ1£»4£ŗ3£»4£ŗ11£»30g/mol£®
µćĘĄ ±¾Ģāæ¼²éĮĖ°¢·üŁ¤µĀĀŽ¶ØĀɼ°ĘäĶĘĀŪµÄ×ŪŗĻÓ¦ÓĆ£¬ĢāÄæÄѶČÖŠµČ£¬×¢ŅāÕĘĪÕ°¢·üŁ¤µĀĀŽ¶ØĀÉÄŚČŻ£¬ŹģĮ·ÕĘĪÕĪļÖŹµÄĮæÓėĘäĖüĪļĄķĮæÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£¬ŹŌĢāÖŖŹ¶µć½Ļ¶ą”¢¼ĘĖćĮæ½Ļ“󣬳ä·Öæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦¼°»Æѧ¼ĘĖćÄÜĮ¦£®


 »ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
»ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
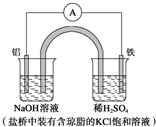
| A£® | Fe×÷Õż¼«£¬·¢ÉśŃõ»Æ·“Ó¦ | |
| B£® | øŗ¼«·“Ó¦£ŗAl-3e-+3OH-ØTAl£ØOH£©3”ż | |
| C£® | ¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬Ź¢ÓŠĻ”ĮņĖįČÜŅŗµÄ±ÖŠpH²»±ä | |
| D£® | ŃĪĒÅÖŠµÄCl-Ļņ×ó±ßÉÕ±ÖŠŅĘ¶Æ£¬Ź¹øĆÉÕ±ÖŠČÜŅŗ±£³ÖµēÖŠŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ś¢Ū¢Ž | B£® | ¢Ł¢Ū¢Ž | C£® | ¢Ł¢Ü¢Ż | D£® | ¢Ś¢Ū¢Ü¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
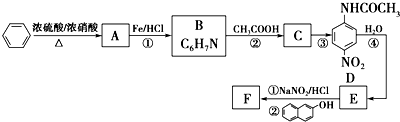
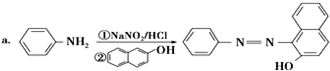
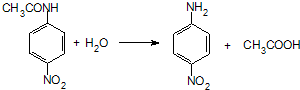 £®
£® £»Éč¼Ę·“Ó¦¢ŚŗĶ¢ÜµÄÄæµÄŹĒ±£»¤°±»ł²»±»Ńõ»Æ£®
£»Éč¼Ę·“Ó¦¢ŚŗĶ¢ÜµÄÄæµÄŹĒ±£»¤°±»ł²»±»Ńõ»Æ£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
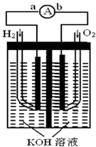 ĒāŃõČ¼ĮĻµē³ŲŹĒ·ūŗĻĀĢÉ«»ÆѧĄķÄīµÄŠĀŠĶ·¢µē×°ÖĆ£®ČēĶ¼ĪŖµē³ŲŹ¾ŅāĶ¼£¬øƵē³Ųµē¼«±ķĆę¶ĘŅ»²ćĻøŠ”µÄ²¬·Ū£¬²¬Īüø½ĘųĢåµÄÄÜĮ¦Ē棬ŠŌÖŹĪČ¶Ø£¬Ēė»Ų“š£ŗ
ĒāŃõČ¼ĮĻµē³ŲŹĒ·ūŗĻĀĢÉ«»ÆѧĄķÄīµÄŠĀŠĶ·¢µē×°ÖĆ£®ČēĶ¼ĪŖµē³ŲŹ¾ŅāĶ¼£¬øƵē³Ųµē¼«±ķĆę¶ĘŅ»²ćĻøŠ”µÄ²¬·Ū£¬²¬Īüø½ĘųĢåµÄÄÜĮ¦Ē棬ŠŌÖŹĪČ¶Ø£¬Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
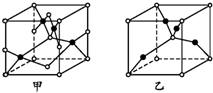 ŅŃÖŖA”¢B”¢C”¢D”¢EĪåÖÖŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĘäÖŠAŌ×ÓĖł“¦µÄÖÜĘŚŹż”¢×åŠņŹż¶¼ÓėĘäŌ×ÓŠņŹżĻąµČ£»BŌ×ÓŗĖĶāµē×ÓÓŠ6ÖÖ²»Ķ¬µÄŌĖ¶ÆדĢ¬£»DŌ×ÓLµē×Ó²ćÉĻÓŠ2¶Ō³É¶Ōµē×Ó£»E+Ō×ÓŗĖĶāÓŠ3²ćµē×ÓĒŅø÷²ć¾ł“¦ÓŚČ«Āś×“Ģ¬£®
ŅŃÖŖA”¢B”¢C”¢D”¢EĪåÖÖŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĘäÖŠAŌ×ÓĖł“¦µÄÖÜĘŚŹż”¢×åŠņŹż¶¼ÓėĘäŌ×ÓŠņŹżĻąµČ£»BŌ×ÓŗĖĶāµē×ÓÓŠ6ÖÖ²»Ķ¬µÄŌĖ¶ÆדĢ¬£»DŌ×ÓLµē×Ó²ćÉĻÓŠ2¶Ō³É¶Ōµē×Ó£»E+Ō×ÓŗĖĶāÓŠ3²ćµē×ÓĒŅø÷²ć¾ł“¦ÓŚČ«Āś×“Ģ¬£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C60Ęų»ÆŗĶI2Éż»ŖæĖ·žµÄ×÷ÓĆĮ¦ĻąĶ¬ | |
| B£® | ¼×Ėį¼×õ„ŗĶŅŅĖįµÄ·Ö×ÓŹ½ĻąĶ¬£¬ĖüĆĒµÄČŪµćĻą½ü | |
| C£® | ĀČ»ÆÄĘŗĶĀČ»ÆĒāČÜÓŚĖ®Ź±£¬ĘĘ»µµÄ»Æѧ¼ü¶¼ŹĒĄė×Ó¼ü | |
| D£® | ÓĆ×÷øßĪĀ½į¹¹ĢՓɲÄĮĻµÄ¹ĢĢåŹĒ·Ö×Ó¾§Ģå |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com