
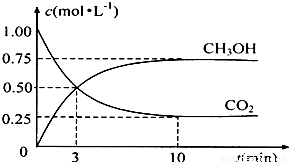
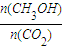 �������______��
�������______��
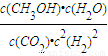 ���ʴ�Ϊ��
���ʴ�Ϊ��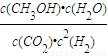 ��
��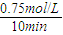 =0.075mol/��L?min�������ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪v��H2��=3v��CO2��=3×0.075mol/��L?min��=0.225 mol/��L?min�����ʴ�Ϊ��0.225 mol/��L?min����
=0.075mol/��L?min�������ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪v��H2��=3v��CO2��=3×0.075mol/��L?min��=0.225 mol/��L?min�����ʴ�Ϊ��0.225 mol/��L?min����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
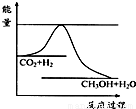
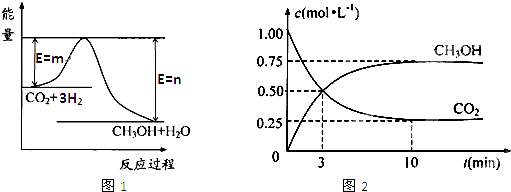
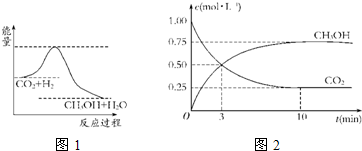
 ������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��
������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g�� CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
| ��� | NaOH��Һ�����/mL | ��������/mL | ��Һ��pH |
| �� | 20.00 | 0.00 | 8 |
| �� | 20.00 | 20.00 | 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
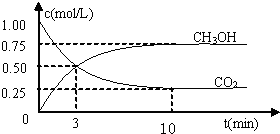 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ��ע
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ��ע| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| 16 |
| 3 |
| 16 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com