
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O),��Ҫ�����������¡�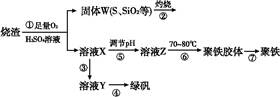
(1)�����̢ڲ���������ͨ��������Һ��,��Һ����ɫ������������
A.Ʒ����Һ B.��ɫʯ����Һ
C.����KMnO4��Һ D.��ˮ
(2)���̢���,FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(3)���̢����������������� ��
(4)���̢���,�����ᾧʱ��ʹ�õ��������ƾ��ơ����ż���,����Ҫ ��
(5)���̢ݵ���pH��ѡ�������Լ��е���������(�����)��
A.ϡ���� B.CaCO3 C.NaOH��Һ
(6)���̢���,����ҺZ���ȵ�70��80 ��,Ŀ������ ��
(7)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص���������,��������ʵ�顣���÷�����ƽ��ȡ2.700 0 g��Ʒ;�ڽ���Ʒ���������������,���������BaCl2��Һ;�۹��ˡ�ϴ�ӡ�����,���صù�������Ϊ3.495 0 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n,��þ�����Ʒ����Ԫ�ص���������Ϊ����������(���������в�����Ԫ�غ���Ԫ��)
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ���仯�������������������й㷺��Ӧ�á�
(1)ͭ�ɲ������·����Ʊ���
����ͭ��Cu2S��O2 2Cu��SO2
2Cu��SO2
ʪ����ͭ��CuSO4��Fe=FeSO4��Cu
�������ַ����У�ͭԪ�ؾ���________(���������ԭ��)��ͭ���ʡ�
(2)ӡˢ��·����ʹ�õ�ͭ��Ҫ�������á�
����һ����FeCl3��Һ����ӡˢ��·���Ʊ�CuCl2��2H2O��ʵ����ģ����չ������£�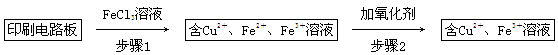

��֤������1����FeCl3��Һ�����ķ�����_________________________________��
�ڲ���2�����ӵ������������˵���______________________________________��
A��HNO3 B��H2O2 C��KMnO4
�۲���3��Ŀ����ʹ��Һ��pH���ߵ�4.2����ʱFe3����ȫ��������ѡ�õġ��Լ�1����________��(д��һ�ּ���)
������Ũ��CuCl2��Һʱ��Ҫ�μ�Ũ���ᣬĿ����________(�û�ѧ����ʽ����ϼ�Ҫ������˵��)���پ���ȴ���ᾧ�����ˣ��õ�CuCl2��2H2O��
����������H2O2��ϡ���Ṳͬ����ӡˢ��·���Ʊ�����ͭʱ�����Ȼ�ѧ����ʽ�ǣ�
Cu(s)��H2O2(l)��H2SO4(aq)=CuSO4(aq)��2H2O(l)����H1����320 kJ��mol��1
��֪��2H2O2(l)=2H2O(l)��O2(g)��H2����196 kJ��mol��1
H2(g)��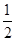 O2(g)=H2O(l)����H3����286 kJ��mol��1
O2(g)=H2O(l)����H3����286 kJ��mol��1
��ӦCu(s)��H2SO4(aq)=CuSO4(aq)��H2(g)�Ħ�H��________��
(3)��ʵ�ַ�ӦCu��H2SO4=CuSO4��H2����������Ϊ��ʵ�ָ�ת����װ���е������ڣ�����缫����(�Cu����C��)��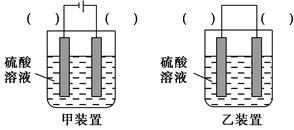
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���㷺Ӧ���ڻ�ѧ��ҵ���ճ������С���ҵ����������(Al2O3��3H2O�ĺ���ԼΪ85%,������ҪΪSiO2��Fe2O3��)ұ�����������������¡�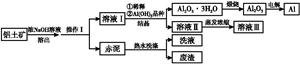
��֪�ݶ�������Al2O3��3H2O�Ļ���ԭ��Ϊ:
Al2O3��3H2O+2NaOH(aq) 2NaAlO2(aq)+4H2O,[Al2O3��3H2OҲ�ɱ�ʾΪ2Al(OH)3]
2NaAlO2(aq)+4H2O,[Al2O3��3H2OҲ�ɱ�ʾΪ2Al(OH)3]
(1)�����������Ϊ��������,�����г���������������,�����е���Ҫ������ ��
(2)Ϊ�������������ܳ����ʿɲ�ȡ����Ч��ʩΪ (��д����)��
(3)�û�ѧƽ�����۽���ϡ����Һ��������Al2O3��3H2O �ᾧ��ԭ���� ��
(4)Ϊ������Al2O3��3H2O,Ҳ������Һ����ͨ�����CO2����,д������Al2O3��3H2O�����ӷ���ʽ: ����
(5)Ϊ�˻��ճ����Һ���ߵ����óɷ�,��ҵ�Ͻ�����ˮϴ�Ӻ��ϴҺ������Һ���ϡ�ͼ�,��ָ������ͼ����һ�����Ƶ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��G����ͼ��ʾ��ת����ϵ����������������ȥ��������A��GΪ���ʣ�D����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬E��F������NaOH��Һ��Ӧ��
��ش��������⣺
��1��д��F�ĵ���ʽ��____________��
��2����C��Һ��D��Ӧ�����ӷ���ʽΪ________________________________________________________________________________________________________________________________________________��
��F��Һ��NaOH��Һ���ȷ�Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________________________________________________________________________________��
��3�����������ӷ���ʽ����C��ҺΪ�������ԣ�________________________________________________________________________________________________________________________________________________��
��F��Һ������Ũ���ɴ�С��˳��Ϊ________________________________________________________________________��
��4����5.4 g AͶ��200 mL 2.0 mol/Lij��Һ����G���ʲ������ҳ�ַ�Ӧ���н���ʣ�࣬�����Һ������________������ţ���
A��HNO3��Һ B��H2SO4��Һ C��NaOH��Һ�� D��HCl��Һ
��5����1 mol N2��3 mol G�����������ݻ�Ϊ2 L��ij�ܱ������н��з�Ӧ����֪�÷�ӦΪ���ȷ�Ӧ��ƽ��ʱ�����D�����ʵ���Ũ��Ϊa mol/L��
�������Ӧ����v��G����1.2 mol/��L��min������v��D����________mol/��L��min����
���������������������£�����ʼʱ����0.5 mol N2��1.5 mol G�ﵽƽ���D�����ʵ���Ũ��________������ڡ���С�ڡ����ڡ��� mol/L��
mol/L��
�۸������µ�ƽ�ⳣ��Ϊ__________________���ú�a�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ��ʯ��Ҫ��������ͭ��Cu2S����������ʯ��SiO2����һ���Ի�ͭ��ʯΪԭ���Ʊ�����ͭ�Ĺ����������£�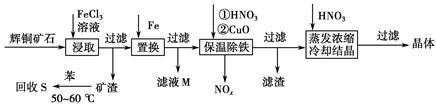
��1��д����ȡ������Cu2S�ܽ�����ӷ���ʽ___________________________��
��2������S�������¶ȿ�����50��60 ��֮�䣬���˹�����͵�ԭ����____________________��____________________��
��3������NOx��������Ϻ�ͨ��ˮ�������������п�ѭ�����õ�һ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ______________________������ҺM�м��루��ͨ�룩����________������ĸ�����ʣ��õ���һ�ֿ�ѭ�����õ����ʡ�
a������������b��������������c���������
��4�����³��������м���CuO��Ŀ����____________________������Ũ��ʱ��Ҫ�����������Һ��pH����������______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����̼�������������ᷴӦ���ж�����̼����ų������������������̼���Ʒ�Ӧ��������̼�����ƺ��Ȼ��ƣ�������ų�������A��B��ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ��ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
��ȡ20mLA��Һ�������л�������B��Һ25mL�����ռ���112mL����״�������塣
��ȡ25mLB��Һ�������л�������A��Һ20mL�����ռ���56mL����״�������塣
��1��д���������������̼���Ʒ�Ӧ��������ų������ӷ���ʽ ��
��2��Ϊʹ�����٢ڷ�Ӧ��ȫ��������� �����ϡ���ᡱ��̼������Һ����A��Һ�����ʵ���Ũ��Ϊ mol��L -1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�սᷨ�������������������£�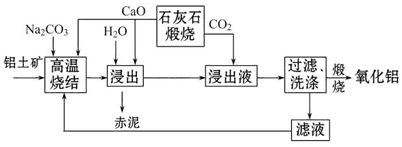
��֪��
����������Ҫ�ɷ�Ϊ��Al2O3��SiO2��Fe2O3��TiO2��
�ڸ����ս�ʱ��Al2O3��Fe2O3��TiO2���ܷ�����Ӧ�ֱ�����NaAlO2��Na2Fe2O4��������ˮ��CaTiO3��
��ش��������⣺
��1��Na2Fe2O4��ˮ�㷢��ˮ�ⷴӦ����Fe��OH��3��д��Na2Fe2O4ˮ��Ļ�ѧ����ʽ____________��
��2������ʱ�ټ���CaO��Ŀ����______________________________________��
��3������Һ�з���������Al��OH��3�����ӷ���ʽΪ____________________________________��
��4����Һ����Ҫ�ɷ���_________��д��ѧʽ������Һѭ��ʹ�õ��ŵ���________ ���δ�һ�㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��100 mL NaOH��Һ������������ͨ��һ������CO2����������Һ����μ���4 mol/L��������Һ��������CO2�����������״���£���������������Һ�����֮��Ĺ�ϵ��ͼ��ʾ������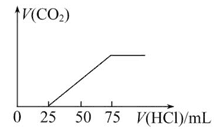
��1��NaOH������CO2�����������Һ�д��ڵ������ǣ�________�������ʵ�����________��
��2���ڼ�����������²�����CO2�������Ϊ����״����________��
��3��ԭNaOH��Һ�����ʵ���Ũ����______________________________________��
����Na2O2��������ˮ���100 mL����Һ��Na2O2__________________________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʶͼ��ͼ����Ҫ�ļ��ܡ���Ҫ��ش��������⣺
��1����̼������Һ�У���μ������ᣬ������������ʵ������������������ͼ��ʾ����OA�η�����Ӧ�����ӷ���ʽΪ________��AB�η�����Ӧ�����ӷ���ʽΪ________��
��2������ͼ�в��������ʯ��ˮ��ͨ��CO2�����ɳ���������CO2����Ĺ�ϵͼ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com