
A”¢B”¢C”¢D 4ÖÖŌŖĖŲ£¬AŌŖĖŲĖł“¦µÄÖÜĘŚŹż”¢Ö÷×åŠņŹż”¢Ō×ÓŠņŹż¾łĻąµČ£»BµÄŌ×Ó°ė¾¶ŹĒĘäĖłŌŚÖ÷×åÖŠ×īŠ”µÄ£¬BµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄ»ÆѧŹ½ĪŖHBO3£»CŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹż±Č“ĪĶā²ćÉŁ2øö£»CµÄŅõĄė×ÓÓėDµÄŃōĄė×Ó¾ßÓŠĻąĶ¬µÄµē×ÓÅŲ¼£¬Į½ŌŖĖŲæÉŠĪ³É»ÆŗĻĪļD2C”£
£Ø1£©BŌŖĖŲµÄĆū³ĘĪŖ__________£»BŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒµŚ________ÖÜĘŚµŚ________×唣
£Ø2£©A”¢BŠĪ³ÉµÄ»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ________”£
£Ø3£©CµÄŌŖĖŲ·ūŗÅĪŖ________£¬CµÄ×īøß¼ŪŃõ»ÆĪļµÄ»ÆѧŹ½ĪŖ____________”£
£Ø4£©DµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄ»ÆѧŹ½ĪŖ________”£
 ĘŚÄ©1¾ķĖŲÖŹ½Ģӿʥ¹Ą¾ķĻµĮŠ“š°ø
ĘŚÄ©1¾ķĖŲÖŹ½Ģӿʥ¹Ą¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C”¢D”¢E”¢FĮłÖÖ¶ĢÖÜĘŚŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£A”¢DĶ¬×壬B”¢CĶ¬ÖÜĘŚ£»BŌ×Ó×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµÄĮ½±¶£»ŌŚÖÜĘŚ±ķÖŠAµÄŌ×Ó°ė¾¶×īŠ”£»CŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ£¬CŹĒF²»Ķ¬ÖÜĘŚµÄĮŚ×åŌŖĖŲ£»EŗĶFµÄŌ×ÓŠņŹżÖ®ŗĶĪŖ30”£ÓÉÉĻŹöĮłÖÖŌŖĖŲÖŠµÄ¼øÖÖŌŖĖŲ×é³ÉµÄ¼×”¢ŅŅ”¢±ū”¢¶””¢Īģ”¢¼ŗĮłÖÖ»ÆŗĻĪļČēĻĀ±ķĖłŹ¾£ŗ
ŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļ
| »ÆŗĻĪļ | ¼× | ŅŅ | ±ū |
| ø÷ŌŖĖŲŌ×Ó øöŹż±Č | N(A)£ŗN(C) £½2£ŗ1 | N(A)£ŗN(C) £½1£ŗ1 | N(B)£ŗN(A) £½1£ŗ4 |
| »ÆŗĻĪļ | ¶” | Īģ | ¼ŗ |
| ø÷ŌŖĖŲŌ×Ó øöŹż±Č | N(D)£ŗN(B)£ŗN(C) £½2£ŗ1£ŗ3 | N(E)£ŗN(F) £½1£ŗ3 | N(B)£ŗN(F) £½1£ŗ4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÖĘĄä¼ĮŹĒŅ»ÖÖŅ×±»Ń¹Ėõ”¢Ņŗ»ÆµÄĘųĢ壬Ņŗ»ÆŗóŌŚ¹ÜÄŚŃ»·£¬Õō·¢Ź±ĪüŹÕČČĮ棬Ź¹»·¾³ĪĀ¶Č½µµĶ£¬“ļµ½ÖĘĄäµÄÄæµÄ”£ČĖĆĒŌų²ÉÓĆŅŅĆŃ”¢NH3”¢CH3ClµČ×÷ÖĘĄäÖĘ£¬µ«ĖüĆĒ²»ŹĒÓŠ¶¾¾ĶŹĒŅ×Č¼£¬ÓŚŹĒæĘѧ¼Ņøł¾ŻŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉĄ“æŖ·¢ŠĀµÄÖĘĄä¼Į”£øł¾ŻŅŃÓŠÖŖŹ¶£¬Ä³Š©ŌŖĖŲ»ÆŗĻĪļµÄŅ×Č¼ŠŌ”¢¶¾ŠŌ±ä»ÆĒ÷ŹĘČēĻĀ£ŗ
(1)Ēā»ÆĪļµÄŅ×Č¼ŠŌ£ŗ
SiH4£¾PH3£¾H2S£¾HCl£¬Ōņ________£¾________£¾H2O£¾HF(ĢīĪļÖŹµÄ»ÆѧŹ½)”£
(2)»ÆŗĻĪļµÄ¶¾ŠŌ£ŗ
PH3£¾NH3£¬CCl4£¾CF4£¬ŌņH2S________H2O£¬CS2________CO2(Ģī”°£¾”±”°£½”±»ņ”°£¼”±)”£
ÓŚŹĒæĘѧ¼ŅĆĒæŖŹ¼°Ń×¢ŅāĮ¦¼ÆÖŠŌŚŗ¬F”¢ClµÄ»ÆŗĻĪļÉĻ”£
(3)ŅŃÖŖCCl4µÄ·ŠµćĪŖ76.8 ”ę£¬CF4µÄ·ŠµćĪŖ£128 ”ę£¬ŠĀµÄÖĘĄä¼ĮµÄ·Šµć·¶Ī§Ó¦½éÓŚ¶žÕßÖ®¼ä£¬¾¹ż½Ļ³¤Ź±¼äµÄ·“ø“ŹµŃ飬·¢ĻÖĮĖÖĘĄä¼ĮCF2Cl2(·śĄū°ŗ)£¬ĘäĖūĄąĖʵÄÖĘĄä¼ĮæÉŅŌŹĒ________”£
(4)Č»¶ųÕāÖÖÖĘĄä¼ĮŌģ³ÉµÄµ±½ńijŅ»»·¾³ĪŹĢāŹĒ_____________________________”£
µ«ĒóÖśÓŚÖÜĘŚ±ķÖŠŌŖĖŲ¼°Ęä»ÆŗĻĪļµÄ______(ĢīŠ“ĻĀĮŠŃ”ĻīµÄ±ąŗÅ)±ä»ÆĒ÷ŹĘæŖ·¢ÖĘĄä¼ĮµÄæĘѧĖ¼Ī¬·½·ØŹĒÖµµĆ½č¼ųµÄ”£
¢Ł¶¾ŠŌ£»¢Ś·Šµć£»¢ŪŅ×Č¼ŠŌ£»¢ÜĖ®ČÜŠŌ£»¢ŻŃÕÉ«
| A£®¢Ł¢Ś¢Ū | B£®¢Ś¢Ü¢Ż | C£®¢Ś¢Ū¢Ü | D£®¢Ł¢Ś¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ģ¼¼°Ęä»ÆŗĻĪļÓ¦ÓĆ¹ć·ŗ”£
I.¹¤ŅµÉĻĄūÓĆCOŗĶĖ®ÕōĘū·“Ó¦ÖĘĒāĘų£¬“ęŌŚŅŌĻĀĘ½ŗā:CO(g)+H2O(g) 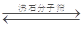 CO2(g)+H2(g)
CO2(g)+H2(g)
£Ø1£©·ŠŹÆ·Ö×ÓÉøÖŠŗ¬ÓŠ¹čŌŖĖŲ£¬ĒėŠ“³ö¹čŌ×Ó½į¹¹Ź¾ŅāĶ¼__________”£
£Ø2£©Ļņ1LŗćČŻĆܱÕČŻĘ÷֊עČĖCOŗĶH2o(g),830”ꏱ²āµĆ²æ·ÖŹż¾ŻČēĻĀ±ķ”£ŌņøĆĪĀ¶ČĻĀ·“Ó¦µÄĘ½ŗā³£
ŹżK=______________”£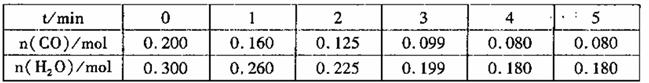
£Ø3£©ĻąĶ¬Ģõ¼žĻĀ£¬Ļņ1LŗćČŻĆܱÕČŻĘ÷ÖŠ£¬Ķ¬Ź±×¢ČĖ1mol CO”¢1mol H2O(g),2molCO2ŗĶ2mo1 H2£¬“ĖŹ±v(Õż ) __________v(Äę)(Ģī”°£¾”±”°£½”±»ņ”°£¼”±)
II.ŅŃÖŖCO(g)+1/2 O2 (g)£½CO2 (g) ”÷H£½Ņ»141 kJ”¤mol-1
2H2(g)+ O2(g)£½2H2O(g) ”÷H£½Ņ»484 kJ”¤mol-1
CH3OH£Ø1£©+3/2O2 (g)£½CO2(g)+2H2O(g) ”÷Hl£½Ņ»726 kJ”¤mol-1
£Ø4£©ĄūÓĆCO”¢H2»ÆŗĻÖʵĆŅŗĢ¬¼×“¼µÄČČ»Æѧ·½³ĢŹ½ĪŖ___________”£
III.Ņ»ÖÖŠĀŠĶĒāŃõČ¼ĮĻµē³Ų¹¤×÷ŌĄķČēĻĀĶ¼ĖłŹ¾
£Ø5£©Š“³öµē¼«AµÄµē¼«·“Ó¦Ź½_____________”£
£Ø6£©ŅŌÉĻŹöµē³Ųµē½ā±„ŗĶŹ³ŃĪĖ®£¬ČōÉś³É0.2mo1 Cl2£¬ŌņÖĮÉŁŠčĶØČĖO2µÄĢå»żĪŖ_____L(±ź×¼×“æö)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)X”¢Y”¢ZŹĒ¶ĢÖÜĘŚČżÖÖŌŖĖŲ£¬ĖüĆĒŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆČēĶ¼ĖłŹ¾”£ŹŌ»Ų“š£ŗ
| | | X |
| | Y | |
| Z | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠ5ÖÖ¶ĢÖÜĘŚŌŖĖŲµÄŌ×ÓŠņŹż°“E”¢D”¢B”¢A”¢CµÄĖ³ŠņŅĄ“ĪŌö“ó£»A”¢CĶ¬ÖÜĘŚ£¬B”¢CĶ¬Ö÷×壻AÓėBæÉŠĪ³ÉĄė×Ó»ÆŗĻĪļA2B£¬A2BÖŠĖłÓŠĮ£×ӵĵē×ÓŹżĻąĶ¬£¬ĒŅµē×Ó×ÜŹżĪŖ30£»DŗĶEæÉŠĪ³É4ŗĖ10µē×ӵķÖ×Ó”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÓƵē×ÓŹ½±ķŹ¾Ąė×Ó»ÆŗĻĪļA2BµÄŠĪ³É¹ż³Ģ£ŗ__________________________________________________________________________”£
£Ø2£©Š“³öĻĀĮŠĪļÖŹµÄµē×ÓŹ½£ŗ
DŌŖĖŲŠĪ³ÉµÄµ„ÖŹ__________£»BÓėEŠĪ³ÉµÄ»ÆŗĻĪļE2B__________£»A”¢B”¢EŠĪ³ÉµÄ»ÆŗĻĪļ__________£»D”¢EŠĪ³ÉµÄ»ÆŗĻĪļ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŖĖŲÖÜĘŚ±ķŹĒѧĻ°»ÆѧµÄÖŲŅŖ¹¤¾ß£¬ĖüŅžŗ¬Šķ¶ąŠÅĻ¢ŗĶ¹ęĀÉ”£ĻĀ±ķĖłĮŠŹĒĪåÖÖ¶ĢÖÜĘŚµÄŌ×Ó°ė¾¶¼°Ö÷ŅŖ»ÆŗĻ¼Ū£ØŅŃÖŖīėµÄŌ×Ó°ė¾¶ĪŖ0.089 nm£©”£
| ŌŖĖŲ“śŗÅ | A | B | C | D | E |
| Ō×Ó°ė¾¶/nm | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 |
| Ö÷ŅŖ»ÆŗĻ¼Ū | £«2 | £«3 | £«6£¬£2 | £1 | £2 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶ĢÖÜĘŚŌŖĖŲA”¢B”¢C”¢DŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆČēĶ¼,E2+ÓėDµÄ¼ņµ„ŅõĄė×ÓÓŠĻąĶ¬µÄµē×Ó²ć½į¹¹,»Ų“šĻĀĮŠĪŹĢā:
(1)ÉčA”¢B”¢C”¢DĖÄÖÖŌŖĖŲµÄŌ×ÓŠņŹżÖ®ŗĶĪŖm,Ōņm”””””””£
A.Ņ»¶ØĪŖĘꏿ
B.Ņ»¶ØĪŖżŹż
C.æÉÄÜĪŖĘꏿ,Ņ²æÉÄÜĪŖżŹż
(2)DŌŖĖŲŌ×ӵēĪĶā²ćµē×ÓŹżµČÓŚĘäĖū²ćµē×ÓŹżÖ®ŗĶ,Ōņ:
¢ŁŠ“³öAŠĪ³ÉµÄ¼ņµ„Ąė×ӵĽį¹¹Ź¾ŅāĶ¼””””””””,ŌŖĖŲDĪ»ÓŚŌŖĖŲÖÜĘŚ±ķµÄµŚ””””””””×唣
¢ŚAŌŖĖŲµÄŅ»ÖÖĒā»ÆĪļ·Ö×ÓÖŠÓŠ6øöŌ×Ó,Ęä½į¹¹¼ņŹ½ĪŖ”””””””””””””””£³£Ń¹298 KŹ±0.2 moløĆĘųĢ¬Ēā»ÆĪļŌŚO2ÖŠĶźČ«Č¼ÉÕ,Éś³ÉĘųĢ¬Aµ„ÖŹŗĶĖ®,·Å³öČČĮæ106.8 kJ,øĆĘųĢ¬Ēā»ÆĪļČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ”” ”””£
¢ŪŠ“³öCµ„ÖŹÓėBµÄ×ī¼ņµ„Ēā»ÆĪļ·“Ó¦µÄ»Æѧ·½³ĢŹ½:
¢ÜŹµŃéÖ¤ŹµAC3ÓėĖ®»į·¢Éś·“Ӧɜ³ÉHNO2ŗĶHF,ŌņAC3ŗĶNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŖĖŲW”¢X”¢Y”¢Z”¢M”¢N¾łĪŖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬ĒŅŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£ŅŃÖŖYŌ×Ó×īĶā²ćµē×ÓŹżÓėŗĖĶāµē×Ó×ÜŹżÖ®±ČĪŖ3”Ć4£¬MŌ×ÓµÄ×īĶā²ćµē×ÓŹżÓė“ĪĶā²ćµē×ÓŹżÖ®±ČĪŖ3”Ć4£¬ĒŅMŌ×ÓµÄÖŹ×ÓŹżŹĒYŌ×ÓµÄ2±¶£»XŌŖĖŲµÄ×īøßÕż¼ŪÓė×īµĶøŗ¼Ū¾ų¶ŌÖµÖ®²īĪŖ2£»N£”¢Z£«”¢W£«µÄ°ė¾¶Öš½„¼õŠ”£»»ÆŗĻĪļWN³£ĪĀĻĀĪŖĘųĢ壬¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)WÓėYæÉ·Ö±šŠĪ³É10µē×ÓŗĶ18µē×ӵķÖ×Ó£¬Š“³öøĆ18µē×Ó·Ö×Ó×Ŗ»Æ³É10µē×Ó·Ö×ӵĻÆѧ·½³ĢŹ½£ŗ_________________________________________”£
(2)ĻĀĶ¼±ķŹ¾ÓÉÉĻŹöĮ½ÖÖŌŖĖŲ×é³ÉµÄĘųĢå·Ö×ÓŌŚŅ»¶ØĢõ¼žĻĀµÄĆܱÕČŻĘ÷ÖŠ³ä·Ö·“Ó¦Ē°ŗóµÄ×Ŗ»Æ¹ŲĻµ£¬ĒėŠ“³öøĆ×Ŗ»Æ¹ż³ĢµÄ»Æѧ·½³ĢŹ½£ŗ_________________”£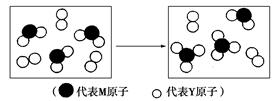
(3)A”¢B¾łĪŖÓÉÉĻŹöĮłÖÖŌŖĖŲÖŠµÄČżÖÖŌŖĖŲ×é³ÉµÄĒæµē½āÖŹ£¬ĒŅ×é³ÉŌŖĖŲµÄŌ×ÓøöŹżÖ®±Č¾łĪŖ1”Ć1”Ć1”£ČōŌŚø÷×ŌµÄĖ®ČÜŅŗÖŠ£¬AÄÜŅÖÖĘĖ®µÄµēĄė£¬BÄÜ“Ł½ųĖ®µÄµēĄė£¬ŌņAµÄ»ÆѧŹ½ĪŖ________£¬BµÄµē×ÓŹ½ŹĒ________”£
(4)XY2ÓėH2O·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com