
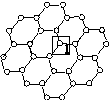
��ʯīϩ[��ͼ(��)��ʾ]��һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ[��ͼ(��)��ʾ]��
 ��
�� 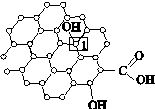
(��)ʯīϩ�ṹ���� ������(��)����ʯīϩ�ṹ (��)
(1)ͼ(��)�У�1��C������C�γɦҼ��ĸ���Ϊ________��
(2)ͼ(��)�У�1��C���ӻ���ʽ��________����C������C�γɵļ���________(�>����<������)ͼ(��)��1��C������C�γɵļ��ǡ�
(3)����ͼ(��)��ʾ������ʯīϩ��ɢ��H2O�У�������ʯīϩ�п���H2O�γ������ԭ����________(��Ԫ�ط���)��
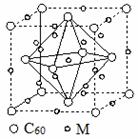
(4)ʯīϩ��ת��Ϊ����ϩ(C60)��ij����M��C60���Ʊ�һ�ֵ��³������ϣ�������ͼ(��)��ʾ��Mԭ��λ�ھ������������ڲ����þ�����Mԭ�ӵĸ���Ϊ________���ò��ϵĻ�ѧʽΪ________��
��A�����������(Se)����(Te)��Ԫ���ڻ������г����ֳ���������̬������A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
(5) H2SeO3��K1��K2�ֱ�Ϊ2.7��10��3��2.5��10��8��H2SeO4��һ��������ȫ���룬K2Ϊ1.2��10��2������ݽṹ�����ʵĹ�ϵ���ͣ�
 ��H2SeO3��H2SeO4��һ������̶ȴ��ڵڶ��������ԭ�� ��
��H2SeO3��H2SeO4��һ������̶ȴ��ڵڶ��������ԭ�� ��
��H2SeO4��H2SeO3����ǿ��ԭ�� ��
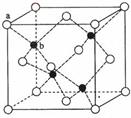
(6)ZnS��ӫ���塢�����ϡ�Ϳ�ϡ����ϵ���ҵ��Ӧ�ù㷺������ZnS����ṹ����ͼ��ʾ���侧���߳�Ϊ540.0pm�����ܶ�Ϊ
g/cm3(��ʽ������)��aλ��S2����bλ��Zn2+֮��ľ���Ϊ pm(��ʽ��ʾ)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ijһ�ݻ�Ϊ V L���ܱ������ڣ���������ʵ�����X��Y����һ���������·������·�Ӧ��X(g)��Y(g) 2Z(g)����H<0
2Z(g)����H<0
(1)�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ______________��
(2)�������¶ȣ���ѧ��Ӧ����________��Z��Ũ��________����ѧƽ�ⳣ��K________(�����������С�����䡱)��
(3)��ͼ�Т��ʾ�÷�Ӧ��t1ʱ�ﵽƽ�⣬��t2ʱ��ı�ij�������������仯�����ߣ�
��ͼ���жϣ���Ӧ������t2 minʱ�����߷����仯��ԭ���� (�����ֱ�ʾ)��
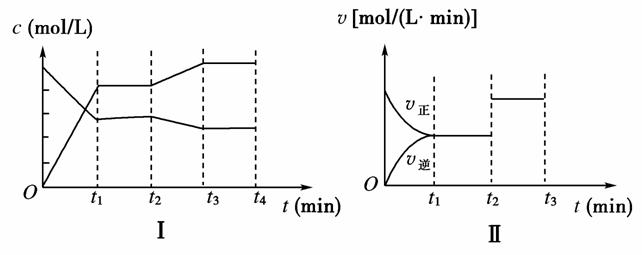
��ͼ���жϣ�t2 min��t3 min�����߱仯��ԭ�������________(����ĸ)��
a�������¶� b�����˴��� c������Z���� d����С�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ȼ�ѧ����ʽ��д��ȷ��������)��
A.2SO2��O2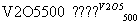 2SO3����H����196.6 kJ��mol��1
2SO3����H����196.6 kJ��mol��1
B.H2g)��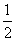 O2g)===H2Ol)����H����285.8 kJ��mol��1
O2g)===H2Ol)����H����285.8 kJ��mol��1
C.2H2g)��O2g)===2H2Ol)����H����571.6 kJ
D.Cs)��O2g)===CO2g)����H����393.5 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ĵð������������ಡ��ҩ�����������һ�ֺϳ�·��(���巴Ӧ�����Ͳ����Լ���)��
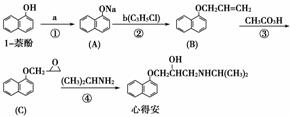
�ش��������⣺
(1)�Լ�a��________���Լ�b�Ľṹ��ʽΪ________��b�й����ŵ�������________��
(2)�۵ķ�Ӧ������________��
(3)�ĵð��ķ���ʽΪ________��
(4)�Լ�b���ɱ��龭������Ӧ�ϳɣ�
C3H8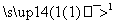 X
X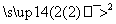 Y
Y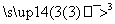 �Լ�b
�Լ�b
��Ӧ1���Լ�������Ϊ________����Ӧ2�Ļ�ѧ����ʽΪ______________�� ��Ӧ3�ķ�Ӧ������________��
(5)���㻯����D��1���ӵ�ͬ���칹�壬������������������ţ��ܷ���������Ӧ��D�ܱ� KMnO4������Һ������E(C2H4O2)�ͷ��㻯����F(C8H6O4)��E��F��̼��������Һ��Ӧ���ܷų�CO2���壬F�����ϵ�һ��������ֻ��һ�֡�D�Ľṹ��ʽΪ________����F����һ��������Ļ�ѧ����ʽΪ________________���ò����������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�к��������������е������֣�K+��Mg2+��Fe3+��Fe2+��
CO32����NO3����SO42����I����SiO32����Cl�������ʵ���Ũ����ͬ��ijͬѧ��̽������Һ����ɣ�����������ʵ�飺
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ������۲쵽dz��ɫ���棻
����ȡԭ��Һ����������������ɫ�������ɣ���������������ɺ���ɫ����ʱ��Һ��ɫ������������ɣ�
��ȡ��Ӧ�����Һ��������֧�Թ��м���BaCl2��Һ���а�ɫ�������ɣ��ٵμ�
KSCN��Һ�ϲ���Һ��죻�ڶ�֧�Թ��м���CCl4��������ú���Һ�ֲ㣬��
������Ϻ�ɫ������˵����ȷ����
A��ԭ��Һ�п϶�����Mg2+��SiO32��
B�����������ɫ������ܺ���CO2��ԭ��Һ�п��ܺ���CO32��
C��ԭ��Һ��K+��Fe2+��NO3����I����SO42�������������
D��ԭ��Һ��һ������Mg2+��Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����(����)
A���ڱ���£�11.2 L NO��11.2 L O2��Ϻ�����������Ϊ0.75NA
B�����³�ѹ�£�16 g O3�����ĵ�����Ϊ8NA
C��0.1 mol Na2O2�������0.4NA������
D����������������Һ��Ӧ����1 mol����ʱ��ת�Ƶĵ�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ԫ���ж��ֻ��ϼۣ����γɶ��ֻ����
(1)��ת���������ȥ��ȼú�����ж�������ķ���֮һ��������������ͼ��ʾ��д���������з�����Ӧ�Ļ�ѧ����ʽ��________________________________________________��
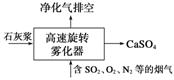
(2)��֪NaHSO3��Һ�������ԣ���ԭ�������ӷ���ʽ��ʾΪ____________��
(3)�밴��Ũ���ɴ�С��˳������0.1 mol·L��1 Na2SO3 ��Һ�е�����________��Na2SO3��Һ�����ڿ�����һ��ʱ�����Һ��pH________(���������С�����䡱)��
(4)ijͬѧ�ڳ������������ʵ������̽��Na2S2O3�Ļ�ѧ���ʡ�
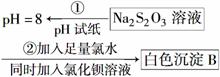
ʵ��ٿ�˵��________(����ĸ)��
A����Na2S2O3��Һ��ˮ�����c(OH��)��10��8 mol·L��1
B��H2S2O3��һ������
C��Na2S2O3��һ���������
D��Na2S2O3�ܷ���ˮ�⣬�����ӷ���ʽΪS2O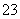 ��2H2O===H2S2O3��2OH��
��2H2O===H2S2O3��2OH��
ʵ���˵��Na2S2O3����________�ԡ�д��������Ӧ�����ӷ���ʽ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������ڣ��������·�ӦN2+3H2 2NH3��
2NH3��
(1)����Ӧ��ijʱ��tʱ��n(N2)=13 mol�� n(NH3)=6 mol��a=_______��
(2)��Ӧ����ƽ��ʱ������������Ϊ716.8 L(��״��)����NH3�ĺ���(�������)Ϊ25%������ƽ��ʱNH3�����ʵ���_________��
(3)���������a��b=_________��
(4)ƽ����������n(N2)��n(H2)��n(NH3)=________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com