
| ŹµŃé ±ąŗÅ | NaOHČÜŅŗµÄÅØ¶Č£Ømol/L£© | µĪ¶ØĶź³ÉŹ±£¬NaOHČÜŅŗµĪČėµÄĢå»ż£ØmL£© | “ż²āHClČÜŅŗµÄĢå»ż£ØmL£© |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |

·ÖĪö £Ø1£©øł¾ŻÖøŹ¾¼ĮĪŖ·ÓĢŖ£¬µĪ¶Ø½įŹųĒ°ČÜŅŗĪŖĪŽÉ«£¬µĪ¶Ø½įŹųŹ±ČÜŅŗ±ä³ÉŗģÉ«½ųŠŠÅŠ¶ĻµĪ¶ØÖÕµć£»
£Ø2£©øł¾ŻµĪ¶ØĻūŗĵıź×¼ŅŗµÄĢå»żÅŠ¶ĻŹż¾ŻµÄÓŠŠ§ŠŌ£¬Č»ŗó¼ĘĖć³ö±ź×¼ŅŗµÄĘ½¾łĢå»ż£¬×īŗóøł¾Ż±źc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$¼ĘĖć³öøĆŃĪĖįµÄÅØ¶Č£»
£Ø3£©øł¾ŻÕżČ·ÅÅČ„¼īŹ½µĪ¶Ø¹ÜÖŠĘųÅŻµÄ·½·Ø½ųŠŠ·ÖĪö£»
£Ø4£©øł¾Ż²Ł×÷¶Ōc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$µÄÓ°Ļģ·ÖĪöÄܹ»µ¼ÖĀ²ā¶Ø½į¹ūĘ«øßµÄŃ”Ļī£®
½ā“š ½ā£ŗ£Ø1£©µĪ¶Ø½įŹųĒ°ŃĪĖįÖŠµĪČė·ÓĢŖ£¬ČÜŅŗĪŖĪŽÉ«£¬µĪ¶Ø½įŹųŹ±ĒāŃõ»ÆÄĘ¹żĮ棬ČÜŅŗ±ä³ÉŗģÉ«£¬ĖłŅŌµĪ¶ØÖÕµćĻÖĻóĪŖ£ŗ×īŗóŅ»µĪĒāŃõ»ÆÄĘČÜŅŗ¼ÓČė£¬ČÜŅŗÓÉĪŽÉ«Ē”ŗƱä³ÉĒ³ŗģÉ«£¬
¹Ź“š°øĪŖ£ŗ×īŗóŅ»µĪĒāŃõ»ÆÄĘČÜŅŗ¼ÓČė£¬ČÜŅŗÓÉĪŽÉ«Ē”ŗƱä³ÉĒ³ŗģÉ«£Ø“šÓÉĪŽÉ«±ä³ÉŗģÉ«Ņ²æÉ£©£»
£Ø2£©Čż“ĪµĪ¶ØŹż¾Ż¶¼ŹĒÓŠŠ§µÄ£¬Ļūŗıź×¼ŅŗµÄĘ½¾łĢå»żĪŖ£ŗ$\frac{22.62+22.72+22.80}{3}$mL=22.713mL£¬
øĆŃĪĖįµÄÅضČĪŖ£ŗc£ØHCl£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$=$\frac{0.1mol/L”Į0.022713L}{0.02L}$”Ö0.11mol/L£¬
¹Ź“š°øĪŖ£ŗ0.11mol/L£»
£Ø3£©¼īŹ½µĪ¶Ø¹Ü×°ĀśČÜŅŗŗó£¬ÓĆÄ“ÖøŗĶŹ³ÖøÄĆ×”²£Į§ĒņĖłŌŚ²æĪ»²¢Ź¹Čé½ŗ¹ÜĻņÉĻĶäĒś£¬³öæŚ¹ÜŠ±ĻņÉĻ£¬Č»ŗóŌŚ²£Į§Ēņ²æĪ»²ąĆęŃøĖŁÄóĻšĘ¤¹Ü£¬Ź¹ČÜŅŗ“Ó¹ÜæŚÅē³ö£¬ĖłŅŌ±ūÕżČ·£¬
¹Ź“š°øĪŖ£ŗ±ū£»
£Ø4£©A”¢µĪ¶ØÖÕµć¶ĮŹżŹ±ø©ŹÓ¶ĮŹż£¬µ¼ÖĀĻūŗıź×¼ŅŗµÄĢå»ż¶ĮŹżĘ«µĶ£¬øł¾Żc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«µĶ£¬¹ŹA“ķĪó£»
B”¢ĖįŹ½µĪ¶Ø¹ÜŹ¹ÓĆĒ°£¬Ė®Ļ“ŗóĪ“ÓĆ“ż²āŃĪĖįČÜŅŗČóĻ“£¬µ¼ÖĀ“ż²āŅŗÅØ¶Č¼õŠ”£¬Ļūŗĵıź×¼ŅŗĢå»ż¼õŠ”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$¼ĘæÉÖŖ£¬²ā¶Ø½į¹ūĘ«µĶ£¬¹ŹB“ķĪó£»
C”¢×¶ŠĪĘæĖ®Ļ“ŗóĪ“øÉŌļ£¬¶Ō±ź×¼ŅŗµÄĪļÖŹµÄĮæ²»Ó°Ļģ£¬µĪ¶ØŹ±¶Ō±ź×¼ŅŗµÄĢå»żĆ»ÓŠÓ°Ļģ£¬øł¾Żc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$¼ĘæÉÖŖ£¬²ā¶Ø½į¹ū²»±ä£¬¹ŹC“ķĪó£»
D”¢ÅäÖĘNaOH±ź×¼ČÜŅŗŹ±£¬Ć»ÓŠµČČܽāŅŗ½µÖĮŹŅĪĀ¾Ķ×ŖŅĘÖĮČŻĮæĘæÖŠ£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$æÉÖŖ£¬“ż²āŅŗÅضČĘ«µĶ£¬¹ŹD“ķĪó£»
E£®ÅäÖĘNaOH±ź×¼ČÜŅŗŹ±£¬¶ØČŻŹ±ŃöŹÓČŻĮæĘæµÄæĢ¶ČĻߣ¬±ź×¼ČÜŅŗµÄÅضČĘ«Š”£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$æÉÖŖ£¬“ż²āŅŗÅضČĘ«“󣬹ŹEÕżČ·£»
F”¢¼īŹ½µĪ¶Ø¹Ü¼ā×ģ²æ·ÖÓŠĘųÅŻ£¬µĪ¶ØŗóĻūŹ§£¬µ¼ÖĀĻūŗĵıź×¼ŅŗĢå»ż¶ĮŹżĘ«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c±ź×¼”ĮV±ź×¼}{V“ż²ā}$¼ĘæÉÖŖ£¬²ā¶Ø½į¹ūĘ«øߣ¬¹ŹFÕżČ·£»
¹ŹŃ”EF£®
µćĘĄ ±¾Ģāæ¼²éĮĖÖŠŗĶµĪ¶Ø¼°Īó²ī·ÖĪö£¬ŅŖĒóŃ”ŌńÕĘĪÕÖŠŗĶµĪ¶ØµÄ²Ł×÷·½·Ø¼°Ķź³É·ÖĪöµÄ·½·Ø£¬ŹŌĢā»ł“”ŠŌĒ棬Ģł½üøßæ¼£»øĆĢāÄŃŅ׏ŹÖŠ£¬×¢ÖŲĮé»īŠŌ£¬²ąÖŲ¶ŌѧɜÄÜĮ¦µÄÅąŃųŗĶ½āĢā·½·ØµÄÖøµ¼ŗĶѵĮ·£¬ÓŠĄūÓŚÅąŃųѧɜµÄĀß¼Ė¼Ī¬ÄÜĮ¦ŗĶŃĻ½÷µÄ¹ę·¶ŹµŃé²Ł×÷ÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ¶ŌŹå¶”»ł±½·Ó£Ø
¶ŌŹå¶”»ł±½·Ó£Ø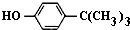 £©¹¤ŅµÓĆĶ¾¹ć·ŗ£¬æÉÓĆÓŚÉś²śÓĶČÜŠŌ·ÓČ©Ź÷Ö¬”¢ĪČ¶Ø¼ĮŗĶĻćĮĻµČ£®ŹµŃéŹŅŅŌ±½·Ó”¢Źå¶”»łĀČ[£ØCH3£©3CCl]µČĪŖŌĮĻÖʱø¶ŌŹå¶”»ł±½·Ó£®
£©¹¤ŅµÓĆĶ¾¹ć·ŗ£¬æÉÓĆÓŚÉś²śÓĶČÜŠŌ·ÓČ©Ź÷Ö¬”¢ĪČ¶Ø¼ĮŗĶĻćĮĻµČ£®ŹµŃéŹŅŅŌ±½·Ó”¢Źå¶”»łĀČ[£ØCH3£©3CCl]µČĪŖŌĮĻÖʱø¶ŌŹå¶”»ł±½·Ó£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
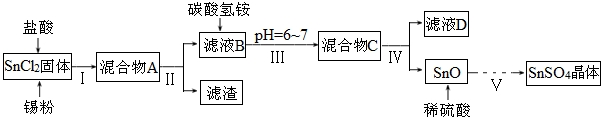
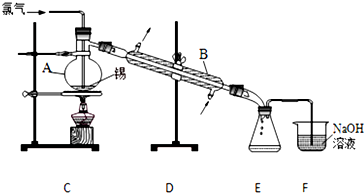
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| µĪ¶Ø“ĪŹż ŹµŃ鏿¾Ż | 1 | 2 | 3 | 4 |
| V£Ø“ż²āŅŗ£©/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V£ØNaOH£©/mL£Ø³õ¶ĮŹż£© | 0.00 | 0.200 | 0.10 | 0.00 |
| V£ØNaOH£©/mL£ØÖÕ¶ĮŹż£© | 14.95 | 15.20 | 15.15 | 16.95 |
| V£ØNaOH£©/mL£ØĻūŗÄ£© | 14.95 | 15.00 | 15.05 | 16.95 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ij»ÆѧѧĻ°Š”×éµÄŃŠ¾ææĪĢāŹĒ£ŗĢ½¾æ²ā¶Ø²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©ÖŠxµÄÖµ£®øĆ×éĶ¬Ń§Ķعż²éŌÄ׏ĮĻ²éŃ°µĆ£¬²ŻĖįŅ×ČÜÓŚĖ®£¬Ė®ČÜŅŗæÉŅŌÓĆĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£ŗ
ij»ÆѧѧĻ°Š”×éµÄŃŠ¾ææĪĢāŹĒ£ŗĢ½¾æ²ā¶Ø²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©ÖŠxµÄÖµ£®øĆ×éĶ¬Ń§Ķعż²éŌÄ׏ĮĻ²éŃ°µĆ£¬²ŻĖįŅ×ČÜÓŚĖ®£¬Ė®ČÜŅŗæÉŅŌÓĆĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
 ij»ÆѧŹµŃ銔×éĻėŅŖĮĖ½āŹŠ³”ÉĻĖłŹŪŹ³ÓĆ°×“×£ØÖ÷ŅŖŹĒ“×ĖįµÄĖ®ČÜŅŗ£©µÄ×¼Č·ÅØ¶Č£¬ĻÖ“ÓŹŠ³”ÉĻĀņĄ“Ņ»ĘæÄ³Ę·ÅĘŹ³ÓĆ°×“×£¬ŌŚŹµŃéŹŅÖŠÓƱź×¼NaOHČÜŅŗ¶ŌĘä½ųŠŠµĪ¶Ø£®ĻĀ±ķŹĒ4ÖÖ³£¼ūÖøŹ¾¼ĮµÄ±äÉ«·¶Ī§£ŗ
ij»ÆѧŹµŃ銔×éĻėŅŖĮĖ½āŹŠ³”ÉĻĖłŹŪŹ³ÓĆ°×“×£ØÖ÷ŅŖŹĒ“×ĖįµÄĖ®ČÜŅŗ£©µÄ×¼Č·ÅØ¶Č£¬ĻÖ“ÓŹŠ³”ÉĻĀņĄ“Ņ»ĘæÄ³Ę·ÅĘŹ³ÓĆ°×“×£¬ŌŚŹµŃéŹŅÖŠÓƱź×¼NaOHČÜŅŗ¶ŌĘä½ųŠŠµĪ¶Ø£®ĻĀ±ķŹĒ4ÖÖ³£¼ūÖøŹ¾¼ĮµÄ±äÉ«·¶Ī§£ŗ| ÖøŹ¾¼Į | ŹÆČļ | ¼×»ł³Č | ¼×»łŗģ | ·ÓĢŖ |
| ±äÉ«·¶Ī§£ØpH£© | 5.0”«8.0 | 3.1”«4.4 | 4.4”«6.2 | 8.2”«10.0 |
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄNaOHČÜŅŗĢå»ż/mL | 26.02 | 25.35 | 25.30 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚĘųĢå·¢Éś×°ÖĆÉĻµćČ¼ĒāĘųµČĘųĢåŹ±£¬±ŲŠėĻČ¼ģŃéĘųĢåµÄ“æ¶Č | |
| B£® | ¼ÓČČ×ĘÉÕŗóµÄŪį¹ų·ÅÖĆŌŚŹµŃéץÉĻĄäČ“ÖĮŹŅĪĀ | |
| C£® | Čō²»Š”ŠÄ“ņ·¾Ę¾«µĘŹ¹¾Ę¾«×Å»šŹ±£¬Ó¦ÓĆŹŖÄز¼øĒĆš | |
| D£® | ÕōĮó²Ł×÷¹ż³ĢÖŠ£¬Čō·¢ĻÖĶü¼Ó·ŠŹÆ£¬Ó¦Į¢¼“Ķ£Ö¹¼ÓČČ£¬“żÉÕĘæĄäČ“ŗóŌŁ¼ÓČė·ŠŹÆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
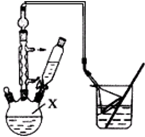
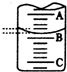
 ÓĆŅ»ÕÅŅŃ³żČ„±ķĆęŃõ»ÆĤµÄĀĮ²½ō½ō°ü¹üŌŚŹŌ¹ÜĶā±Ś£ØČēĶ¼£©£¬½«ŹŌ¹Ü½žČėĻõĖį¹ÆČÜŅŗÖŠ£¬Ę¬æĢČ”³ö£¬Č»ŗóÖĆÓŚæÕĘųÖŠ£¬²»¾ĆĀĮ²±ķĆęÉś³ö”°°×Ć«”±£¬ŗģÄ«Ė®ÖłÓŅ¶ĖÉĻÉż£®øł¾ŻŹµŃéĻÖĻóÅŠ¶ĻĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©
ÓĆŅ»ÕÅŅŃ³żČ„±ķĆęŃõ»ÆĤµÄĀĮ²½ō½ō°ü¹üŌŚŹŌ¹ÜĶā±Ś£ØČēĶ¼£©£¬½«ŹŌ¹Ü½žČėĻõĖį¹ÆČÜŅŗÖŠ£¬Ę¬æĢČ”³ö£¬Č»ŗóÖĆÓŚæÕĘųÖŠ£¬²»¾ĆĀĮ²±ķĆęÉś³ö”°°×Ć«”±£¬ŗģÄ«Ė®ÖłÓŅ¶ĖÉĻÉż£®øł¾ŻŹµŃéĻÖĻóÅŠ¶ĻĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©| A£® | ŹµŃéÖŠ·¢ÉśµÄ·“Ó¦¶¼ŹĒ»ÆŗĻ·“Ó¦ | B£® | ĀĮŹĒŅ»ÖÖ½Ļ»īĘĆµÄ½šŹō | ||
| C£® | ĀĮÓėŃõĘų·“Ó¦·Å³ö“óĮæµÄČČĮæ | D£® | ĀĮʬÉĻÉś³ÉµÄ°×Ć«ŹĒŃõ»ÆĀĮ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com