
| �ζ����� | �������mL | NaOH��Һ���������mL�� | ����NaOH��Һ��ƽ�������mL�� | |
| �ζ�ǰ | �ζ��� | |||
| 1 | 20.00 | 0.00 | 16.26 | |
| 2 | 20.00 | 0.00 | 16.30 | |
| 3 | 20.00 | 0.02 | 16.24 | |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ���ô�ͼװ�ÿɴ��Ȼ�����Һ��ֱ�������ᾧ����Ȼ������� |
B�� ���ô�ͼװ�ÿɷ���ʯ�ͣ��õ����͡�ú�ͺͲ��͵ȸ������ |
C��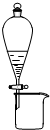 ���ô�ͼװ�ÿɷ���CH3CH2OH��CH3COOC2H5���Һ |
D�� ���ô�ͼװ�ÿɽ�������к͵ζ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����״���£�11.2L�Ҵ��������ǻ���Ϊ0.5 NA |
| B�����³�ѹ�£�4.6g NO2��N2O4������к��е�ԭ������Ϊ0.3 NA |
| C��1mol Cl2������Ӧʱ��ת�Ƶĵ�����һ����2NA |
| D�������£�1L pH=12��Ba��OH��2��Һ�к��е�OH-������Ϊ0.02NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ྻ�����ֽ���ƬX��Y��Z�ֱ�����ڽ���ʳ����Һ����ֽ�ϲ�ѹ������ͼ����ÿ��ʵ��ʱ����ѹ��ָ���ƫ�Ʒ���Ͷ������±�����֪�������缫�Ľ��������������Խ�����ѹԽ��X��Y��Z��ͭ���ֽ���������˵��������ȷ���ǣ�������
���ྻ�����ֽ���ƬX��Y��Z�ֱ�����ڽ���ʳ����Һ����ֽ�ϲ�ѹ������ͼ����ÿ��ʵ��ʱ����ѹ��ָ���ƫ�Ʒ���Ͷ������±�����֪�������缫�Ľ��������������Խ�����ѹԽ��X��Y��Z��ͭ���ֽ���������˵��������ȷ���ǣ�������| ����Ƭ | �������� | ��ѹ��V�� |
| X | X��Cu | +0.78 |
| Y | Cu��Y | -0.15 |
| Z | Z��Cu | +1.35 |
| A��Z����������������������������������Y���� |
| B��Y�������ܴ�������Һ���û������� |
| C�����ֽ����Ļ�����˳��Ϊ��Z��X��Y |
| D��X��Y�ܹ��ɵ�ѹ����ԭ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��7.8g Na2O2�к��е���������ĿΪ0.4NA |
| B��1mol�������к���̼̼˫����ĿΪ3NA |
| C����״���£�����������ΪNA��NH3��HCl�����Ϻ�����ԼΪ22.4L |
| D��16g CH4��18g NH4+������������Ϊ10NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com