
��t���£���1 L�����ܱ�ϵ�з�����Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H��0������Ӧ�����ʼŨ�ȷֱ�Ϊ��c(CO)��5.00 mol/L��c(H2O)��6.00 mol/L��c(CO2)��0��c(H2)��1.50 mol/L���ﵽƽ���c(CO)Ũ��Ϊ2.00 mol/L��
CO2(g)��H2(g)����H��0������Ӧ�����ʼŨ�ȷֱ�Ϊ��c(CO)��5.00 mol/L��c(H2O)��6.00 mol/L��c(CO2)��0��c(H2)��1.50 mol/L���ﵽƽ���c(CO)Ũ��Ϊ2.00 mol/L��
��
(1)����ƽ���������ƽ��Ħ��������
(2)����t���£���Ӧ�����ʼŨ�ȷֱ�Ϊ��c(CO)��3.00 mol/L��c(H2O)��3.00 mol/L����������ʼŨ�Ⱦ�Ϊ0����ﵽƽ��ʱH2Oת���ʣ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��1����һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g)+H2(g)![]() CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���¡���ش��������⣺
CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���¡���ش��������⣺
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽK = �����ϱ����ݿɵã��÷�ӦΪ ��Ӧ��������ȡ����ȡ���
��800�棬�̶��������ܱ������У����������ʼŨ��Ϊc(CO)=0.01 mol��L��1��c(H2O)=0.03mol��L��1��c(CO2)=0.01 mol��L��1��c(H2)=0.05 mol��L��1����Ӧ��ʼʱ��H2O���������ʱ��������� (�����С������ȷ����)
��830�棬��1 L�Ĺ̶��������ܱ������з���2 mol CO2��1 mol H2��ƽ���CO2��ת����Ϊ ��
��2��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1L���ܱ������У�����1mol CO2��3mol H2����500���·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
��ƽ��ʱCH3OH���������wΪ ��
�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����
| ���� | ʵ��1 | ʵ��2 | ʵ��3 |
| ��Ӧ��Ͷ������ʼ̬�� | 1mol CO2��3mol H2 | 1mol CH3OH��1mol H2O | 2mol CH3OH��2mol H2O |
| CH3OH��ƽ��Ũ��/mol��L��1 | C1 | C2 | C3 |
| ��Ӧ�������仯 | �ų� x kJ | ����y kJ | ����z kJ |
| ��ϵѹǿ/Pa | P1 | P2 | P3 |
| ��Ӧ��ת���� | a1 | a2 | a3 |
A��2 C1>C3 B��x+y=49.0 C��2P2<P3
D����a1+a3��<1 E��2P1>P3 F��a1= a2
����һ��װ�п��ƶ������������н���������Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����Ӧ�ﵽƽ����CH3OH�����ʵ���Ϊa mol�����������ڵ��¶Ⱥ�ѹǿ���䣬��ƽ����ϵ��ͨ��������H2���ٴδﵽƽ����CH3OH�����ʵ���Ϊb mol����Ƚ�a��b�Ĵ�С ��
CH3OH(g)+H2O(g)����Ӧ�ﵽƽ����CH3OH�����ʵ���Ϊa mol�����������ڵ��¶Ⱥ�ѹǿ���䣬��ƽ����ϵ��ͨ��������H2���ٴδﵽƽ����CH3OH�����ʵ���Ϊb mol����Ƚ�a��b�Ĵ�С ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�������������߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��14�֣�CO2��ת�����л���ʵ��̼ѭ����
��1�������Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ�� CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=��49.0 mol��L-1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0 mol��L-1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��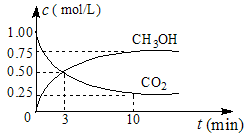
�ٴ�0 min��10 min��v(H2)= mol��(L��min)-1��
����˵��������Ӧ�ﵽƽ��״̬���� ��ѡ���ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1�U1����ͼ�н���㣩
B�������������ѹǿ����ʱ��ı仯���仯
C����λʱ����ÿ����3 mol H2��ͬʱ����1 mol H2O
D��CO2����������ڻ�������б��ֲ���
�����д�ʩ����ʹn (CH3OH)/n (CO2)������� ��ѡ���ţ���
A����H2O(g)����ϵ�з��� B�����º��ݳ���He
C�����º�ѹ����He D�����º����ٳ���1 mol CO2��3 mol H2
��2���ݱ�����һ���������ɶ�����̼�������ϳɶ������ѳ�Ϊ��ʵ��
2CO2(g)+6H2(g)  CH3OCH3(g)+3H2O(g)
CH3OCH3(g)+3H2O(g)
��һ��ѹǿ�£���÷�Ӧ��ʵ���������±���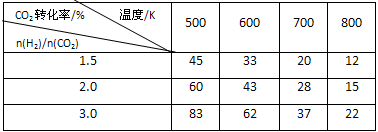
�����������ݻش��������⣺
�ٷ�Ӧ���¶����ߣ�Kֵ �����������С�����䡱����
�������̼��[n��H2��/n��CO2��], Kֵ �����������С�����䡱����
��3��800��ʱ��C(s)��CO2(g) 2CO(g)��ƽ�ⳣ��K��1.64����ͬ�����²��c(CO)��0.20 mol��L��1��c(CO2)��0.05 mol��L��1����ʱ��Ӧ�� ����������桱��������С�
2CO(g)��ƽ�ⳣ��K��1.64����ͬ�����²��c(CO)��0.20 mol��L��1��c(CO2)��0.05 mol��L��1����ʱ��Ӧ�� ����������桱��������С�
��4�����ܱ�������ͨ��1mol H2��1mol CO2����H2(g)+CO2(g)  CO(g)+H2O(g) ��H> 0��Ӧ������Ӧ�ﵽƽ�����������������ʱ���������¶ȣ�������ͼ�л�����(v��)����(v��)��Ӧ������ʱ��t�仯��ʾ��ͼ��
CO(g)+H2O(g) ��H> 0��Ӧ������Ӧ�ﵽƽ�����������������ʱ���������¶ȣ�������ͼ�л�����(v��)����(v��)��Ӧ������ʱ��t�仯��ʾ��ͼ��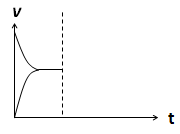
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��T��ʱ����1 L�̶�������ܱ�����M�м���2 mol X��1 mol Y���������·�Ӧ��2X(g)��Y(g)  aZ(g)��W(g) ��H����Q kJ/mol(Q��0)���÷�Ӧ�ﵽƽ��ų�������ΪQ1
kJ������X��ת����Ϊ������ƽ����������¶ȣ���������ƽ����Է���������С��
aZ(g)��W(g) ��H����Q kJ/mol(Q��0)���÷�Ӧ�ﵽƽ��ų�������ΪQ1
kJ������X��ת����Ϊ������ƽ����������¶ȣ���������ƽ����Է���������С��
��ش��������⣺
(1)��ѧ������a��ֵΪ________��
(2)������˵���÷�Ӧ�ﵽ�˻�ѧƽ��״̬����________(�����)��
a��������ѹǿһ��
b��������������ܶ�һ��
c��������Z�ķ�����һ��
d�����������������һ��
(3)ά��T���¶Ȳ��䣬����ʼʱ������M�м���2 mol X��1 mol Y��1 mol Ar(ϡ�����岻���뷴Ӧ)����Ӧ�ﵽƽ���ų���������________kJ��
(4)ά��T���¶Ȳ��䣬����һ����ԭ���������ȵĺ�ѹ����N�м���2 mol X��1 mol Y�����������Ӧ���ﵽƽ�⣬��________(�M����N��)�����еķ�Ӧ�ȴﵽƽ��״̬��������X����������M________N(�����������������)��
(5)��֪���÷�Ӧ��ƽ�ⳣ�����¶ȵı仯��������ʾ��
|
�¶�/�� |
200 |
250 |
300 |
350 |
|
ƽ�ⳣ��K |
9.94 |
5.2 |
1 |
0.5 |
����ij�¶��£�2 mol X��1 mol Y������M�з�Ӧ���ﵽƽ�⣬X��ƽ��ת����Ϊ50%������¶�Ϊ________�档
(6)ά��T���¶Ȳ��䣬����ʼʱ������M�м���4 mol X��6 mol Y����Ӧ�ﵽƽ��ʱ�����ڵķ�����Ŀ����10%����Ӧ�зų�������Ϊ________kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ��У������һ��������ѧ�Ծ� ���ͣ������
��1����һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g)+H2(g)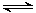 CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���¡���ش��������⣺
CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���¡���ش��������⣺
|
t�� |
700 |
800 |
830 |
1000 |
1200 |
|
K |
0.6 |
0.9 |
1.0 |
1.7 |
2.6 |
�ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽK = �����ϱ����ݿɵã��÷�ӦΪ ��Ӧ��������ȡ����ȡ���
��800�棬�̶��������ܱ������У����������ʼŨ��Ϊc(CO)=0.01 mol��L��1��c(H2O)=0.03 mol��L��1��c(CO2)=0.01 mol��L��1��c(H2)=0.05 mol��L��1����Ӧ��ʼʱ��H2O���������ʱ��������� (�����С������ȷ����)
��830�棬��1 L�Ĺ̶��������ܱ������з���2 mol CO2��1 mol H2��ƽ���CO2��ת����Ϊ ��
��2��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1L���ܱ������У�����1mol CO2��3mol H2����500���·�����Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
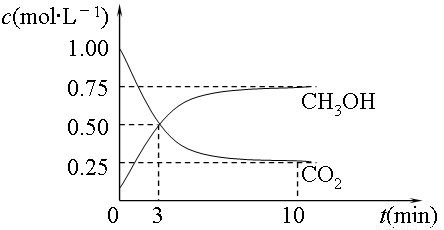
��ƽ��ʱCH3OH���������wΪ ��
�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����
|
���� |
ʵ��1 |
ʵ��2 |
ʵ��3 |
|
��Ӧ��Ͷ������ʼ̬�� |
1mol CO2��3mol H2 |
1mol CH3OH��1mol H2O |
2mol CH3OH��2mol H2O |
|
CH3OH��ƽ��Ũ��/mol��L��1 |
C1 |
C2 |
C3 |
|
��Ӧ�������仯 |
�ų� x kJ |
����y kJ |
����z kJ |
|
��ϵѹǿ/Pa |
P1 |
P2 |
P3 |
|
��Ӧ��ת���� |
a1 |
a2 |
a3 |
A��2 C1>C3 B��x+y=49.0 C��2P2< P3
D����a1+ a3��<1 E��2P1> P3 F��a1= a2
����һ��װ�п��ƶ������������н���������Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)����Ӧ�ﵽƽ����CH3OH�����ʵ���Ϊa mol�����������ڵ��¶Ⱥ�ѹǿ���䣬��ƽ����ϵ��ͨ��������H2���ٴδﵽƽ����CH3OH�����ʵ���Ϊb mol����Ƚ�a��b�Ĵ�С
��
CH3OH(g)+H2O(g)����Ӧ�ﵽƽ����CH3OH�����ʵ���Ϊa mol�����������ڵ��¶Ⱥ�ѹǿ���䣬��ƽ����ϵ��ͨ��������H2���ٴδﵽƽ����CH3OH�����ʵ���Ϊb mol����Ƚ�a��b�Ĵ�С
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com