
���� ��1��������Ũ��Խ����Һ��pHԽС��������ʲ��ֵ��룬ǿ�������ȫ���룬������ʵ�Ũ��Խ��������Ũ��Խ�ݴ�pH��ҺpH��С��
����Һ�У�������Ũ��Խ��ˮ�ĵ���̶�ԽС��ˮ�������c��H+�����������Һ��������Ũ����С��
п������������������ȡ���������к��е������ӵ������ʵ�����
����Ϊ���ᣬ����ʱ�����ȣ�
��2������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��3������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1����100mL 0.1mol/L H2SO4��Һ��������Ũ��Ϊ0.2mol/L��
��100mL 0.15mol/L HCl��Һ��������Ũ��Ϊ0.15mol/L��
��100mL 0.2mol/LCH3COOH��Һ�У�CH3COOH���ֵ��룬��������Ũ��С��0.2mol/L��
��200mL 0.1mol/L CH3COOH��Һ��������Ũ��С��0.1mol/L��
���ݷ�����֪������Һ��������Ũ������Ϊ�٣�����Һ��pH��С��Ϊ�٣�
������Һ�У�������Ũ����С��Ϊ�ܣ���ܶ�ˮ�ĵ�������Ƴ̶���С������Һ��ˮ�����������Ũ�����
��100mL 0.1mol/L H2SO4��Һ���ܹ��ṩ�������ӵ������ʵ���Ϊ��0.1mol/L��2��0.1L=0.02mol��
��100mL 0.15mol/L HCl��Һ���ܹ��ṩ�������ӵ����ʵ���Ϊ��0.15mol��0.1L=0.015mol��
��100mL 0.2mol/LCH3COOH��Һ������ܹ��ṩ�����ӣ�0.2mol/L��0.1L=0.02mol��
��200mL 0.1mol/L CH3COOH��Һ���ܹ��ṩ�������ӵ����ʵ���Ϊ��0.1mol/L��0.1L=0.02mol��
���ݷ�����֪���ܹ��ṩ�����ӵ����ʵ�����С��Ϊ�ڣ���������п��Ӧ�����������ٵ�Ϊ�ڣ�
����Ϊ���ᣬ����ʱ�����ȣ����Ԣٺ͢۷ֱ���100mL 0.2mol/LNaOH��Һ��Ӧ���ų������ٵ��Ǣۣ�
�ʴ�Ϊ���٣��ܣ��ڣ��ۣ�CH3COOH�������ȣ�
��2���ζ�ʱ������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣬����̪������Ϊ��ɫ���ڼ�����Һ����dz��ɫ��
�ʴ�Ϊ�����һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ�Ұ�����ڲ���ɫ��
��3��A���ζ��յ����ʱ���ӣ�����V������ƫС����c�����⣩=$\frac{c��������V������}{V�����⣩}$������c�����⣩ƫС����A����
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ��������Һ��ϡ�ͣ�����Һ�����ʵ���ƫС������V������ƫС����c�����⣩=$\frac{c��������V������}{V�����⣩}$������c�����⣩ƫС����B����
C����ƿˮϴ��δ�������Һ�����ʵ������䣬V���������䣬��c�����⣩=$\frac{c��������V������}{V�����⣩}$������c�����⣩��Ӱ�죬��C����
D����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ������V������ƫ��c�����⣩=$\frac{c��������V������}{V�����⣩}$������c�����⣩ƫ��D��ȷ��
��ѡD��
���� ������Ҫ������������ʵĵ��롢�к͵ζ�����������������ȷ��Һ���������ҺpH�Ĺ�ϵΪ���ؼ�����Ŀ�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬ���ڽ���Ԫ�صĻ��ϼ�Խ�ߣ�Ԫ�صĽ�����Խǿ | |
| B�� | ��������ϸ���һЩп�飬���Լ���������ǵĸ�ʴ | |
| C�� | �ں��� BaSO4 ��������Һ�м��� Na2SO4���壬c��Ba2+�� ���� | |
| D�� | 2NO��g��+2CO��g���TN2��g��+2CO2��g�� �ڳ��������Է����У���÷�Ӧ�ġ�H��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢ� | B�� | �ڢۢܢ� | C�� | �ܢۢڢ� | D�� | �ڢۢ٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
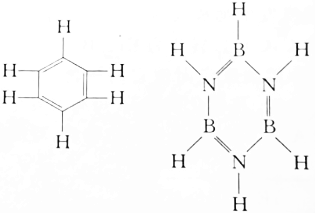
| A�� | ǰ�ߴ��ں��� | B�� | ǰ�ߵ��ں��� | C�� | ǰ��С�ں��� | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�| �ⶨ���� | ����Һ���/mL | ���������/mL | |
| �ζ�ǰ����/mL | �ζ������/mL | ||
| ��һ�� | 25.00 | 0.40 | 20.38 |
| �ڶ��� | 25.00 | 4.00 | 24.02 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
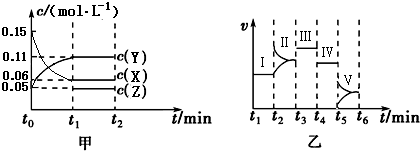
 2Y��g��+Z��g����H=-10akJ/mol������ͼ����ƽ���У�ƽ�ⳣ�������Ǣ���
2Y��g��+Z��g����H=-10akJ/mol������ͼ����ƽ���У�ƽ�ⳣ�������Ǣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
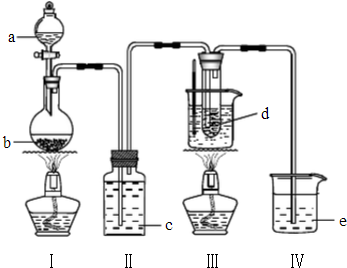 ���������Һ��Ӧʱ���ڵ��º�ϡ����Һ����Ҫ������ClO-��Cl-����75�����Ϻ�Ũ����Һ����Ҫ������ClO-��Cl-���о�С��������ʵ��װ����ȡ����أ�KClO3�����ⶨ�ܶȣ�
���������Һ��Ӧʱ���ڵ��º�ϡ����Һ����Ҫ������ClO-��Cl-����75�����Ϻ�Ũ����Һ����Ҫ������ClO-��Cl-���о�С��������ʵ��װ����ȡ����أ�KClO3�����ⶨ�ܶȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 64gCu��������ʧȥ�ĵ�����һ��Ϊ2NA | |
| B�� | �����£�pH=13�İ�ˮ�У���ˮ�����OH-��Ϊ0.1NA | |
| C�� | �ڱ�״���£�22.4LC4H10�й��ۼ���ĿΪ13NA | |
| D�� | 200mL1mol/LFe2 �� SO4��3 ��Һ�У�SO42-��������0.3NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com