
 �Ħ�HΪ____________________��
�Ħ�HΪ____________________��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| �� �� | ȼ���ȣ�kJ��mol��1�� |
| H2(g) | ��285.8 |
| CO(g) | ��283.0 |
| CH4(g) | ��890.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)
O2(g)  SO3(g) ��H =" �D98.32" kJ��mol��1���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ
SO3(g) ��H =" �D98.32" kJ��mol��1���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ| A��196.64 kJ | B��196.64 kJ��mol��1 | C��<196.64 kJ | D��>196.64 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺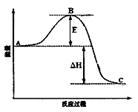
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2H2O(g) �� 2H2(g) �� O2(g)��H1 2H2O(l) �� 2H2(g) �� O2(g)��H2 |
| B��S (g) �� O 2(g) �� SO2(g)��H1 S (s) �� O 2(g) �� SO2(g)��H2 |
| C��2C (s) �� O 2(g) �� 2CO (g)��H1 2C (s) �� 2O 2(g) �� 2CO2 (g)��H2 |
| D��H2(g) �� Cl2(g) �� 2HCl(g)��H1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)==H2O(l) ��H3= ��285 kJ��mol-1
O2(g)==H2O(l) ��H3= ��285 kJ��mol-1| A����243.5 kJ��mol-1 | B����487 kJ��mol-1 | C����1113.5 kJ��mol-1 | D����2227 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 H="-24.8" kJ��mol-1
H="-24.8" kJ��mol-1 H="-47.2" kJ��mol-1
H="-47.2" kJ��mol-1 H="+640.5" kJ��mol-1
H="+640.5" kJ��mol-1| A��-218 kJ��mol-1 | B��-109kJ��mol-1 | C��+218 kJ��mol-1 | D��+109 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��N4����һ�����͵Ļ����� | B��N4��N2��ͬϵ�� |
| C��N4ת��ΪN2�������仯 | D��1molN4����ת��ΪN2�ų�724kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-386kJ��mol-1 | B��+386kJ��mol-1 | C��- | D��+746kJ��mol-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com