
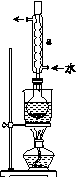
�ں�ۡ��ۡ�����֮�佨����ϵ���ǻ� ѧѧ�����е�˼ά��ʽ������β������ɴ�����Ⱦ����Ҫԭ��֮һ���������������ϰ�װ����ת�����������ʹ������β��ת���������塣���á��ʾ̼ԭ�ӣ��á��ʾ��ԭ�ӣ���
ѧѧ�����е�˼ά��ʽ������β������ɴ�����Ⱦ����Ҫԭ��֮һ���������������ϰ�װ����ת�����������ʹ������β��ת���������塣���á��ʾ̼ԭ�ӣ��á��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
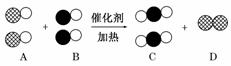
(1)A��B��C�������ʿ��Թ�Ϊһ���������___________________________________��
(2)��C��Ϊ�������D��Ϊ���ʵ�������________________________________
________________________________________________________________________��
(3)�û�ѧ��Ӧ����ʽ��ʾΪ___________________________________________
________________________________________________________________________��
��ѧ�仯���������ĵ�A���ʺ����ɵ�C���ʵ�������Ϊ________��
(4)���۵ĽǶ�ȥ�������õĹ��ڻ�ѧ�仯���й���Ϣ(���һ������)________________________________________________________________________
__________________________________________________ ______________________��
______________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ̽��һ�廷����(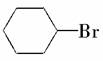 )��NaOH�Ĵ���Һ���ȷ�������ˮ�ⷴӦ������ȥ��Ӧ���ס��ҡ�����λͬѧ�ֱ������������ʵ�鷽����
)��NaOH�Ĵ���Һ���ȷ�������ˮ�ⷴӦ������ȥ��Ӧ���ס��ҡ�����λͬѧ�ֱ������������ʵ�鷽����
�ף���Ӧ���Һ�е���ϡ�����к�NaOH��Ȼ���ٵ���AgNO3��Һ������dz��ɫ�����������֤����������ȥ��Ӧ��
�ң���Ӧ���Һ�е�����ˮ������Һ��ɫ�ܿ���ȥ�����֤����������ȥ��Ӧ��
������Ӧ���Һ�е�������KMnO4��Һ������Һ��ɫ��dz�����֤����������ȥ��Ӧ��
������ȷ����(����)
A����
B����
C����
D������ʵ�鷽��������ȷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
50 gþ��п�����Ļ������������ϡ���ᷴӦ���������εĻ����Һ��Ȼ����ȡ��������ᾧ�þ���(�����ᾧˮ)218 g����Ӧ�е�H2������Ϊ(����)
A��2 g
B��3 g
C��3.5 g
D��4.5 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
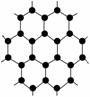 2010��ŵ��������ѧ�����ڱ����״ΰ��������ʯī�Ŀ�ѧ�ҡ�����ʯī��Ϊʯīϩ�����֡�ֻ��һ��̼ԭ�Ӻ��̼��Ƭ����ʯīϩ��������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ�Ӧ��ǰ��ʮ�ֹ��������й���ʯīϩ��������ȷ����(����)
2010��ŵ��������ѧ�����ڱ����״ΰ��������ʯī�Ŀ�ѧ�ҡ�����ʯī��Ϊʯīϩ�����֡�ֻ��һ��̼ԭ�Ӻ��̼��Ƭ����ʯīϩ��������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ�Ӧ��ǰ��ʮ�ֹ��������й���ʯīϩ��������ȷ����(����)
A��ʯīϩ��̼����
B��ʯīϩ��һ���л���
C��ʯīϩ��̼ԭ�ӵĻ��ϼ�Ϊ��3
D��ʯīϩ�ɵ��磬˵�����ǵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ڻ�ѧѧ�Ƶķ�չ������Ҫ�����á����з������������(����)
A�����ݴ������Ԫ����ɣ����������Ϊ���ʺͻ�����
B��������Һ��������ǿ����������ʷ�Ϊǿ����ʡ��������
C�����ݷ�ɢ������ֱ���Ĵ�С������ɢϵ��Ϊ��Һ����Һ�ͽ���
D�����ݷ�Ӧ�е������仯������ѧ��Ӧ��Ϊ�����ϡ��ֽ⡢���ֽ⡢�û�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ�����и���������ָ����Һ��һ���ܴ����������(����)
A��pH��1����Һ�У�Na����K����MnO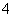 ��CO
��CO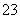
B��c(H��)��1��10��13 mol��L��1����Һ�У�Mg2����Cu2����SO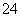 ��NO
��NO
C��0.1 mol��L��1NH4HCO3��Һ�У�K����Na����NO ��Cl��
��Cl��
D��0.1 mol��L��1FeCl3��Һ�У�Fe2����NH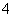 ��SCN����SO
��SCN����SO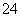
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)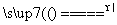 Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O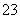 ��I2===S4O
��I2===S4O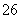 ��2I��
��2I��
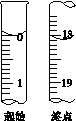
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO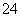 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�
(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
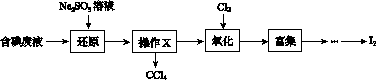
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��
(4)��֪��5SO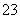 ��2IO
��2IO ��2H��===I2��5SO
��2H��===I2��5SO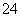 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com