
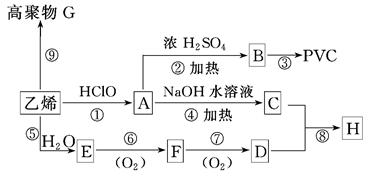
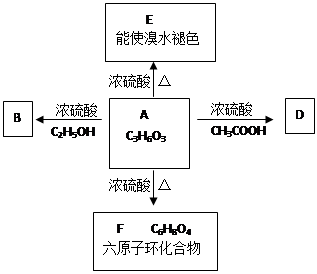
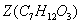 ��д��z��һ�������¾ۺϷ�Ӧ�Ļ�ѧ����ʽ�� ��
��д��z��һ�������¾ۺϷ�Ӧ�Ļ�ѧ����ʽ�� ��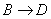 ��
�� �ķ�Ӧ���ͷֱ�Ϊ �� ��
�ķ�Ӧ���ͷֱ�Ϊ �� ��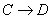 �Ļ�ѧ����ʽΪ ��
�Ļ�ѧ����ʽΪ ��
 ��2�֣���������δ��ƽ����1�֣�
��2�֣���������δ��ƽ����1�֣�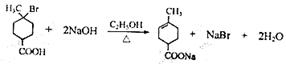 ��2�֣���������δ��ƽ����1�֣�
��2�֣���������δ��ƽ����1�֣� ��2�֣�
��2�֣�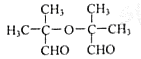 ��2�֣�
��2�֣� ��Z���Ӻ����Ȼ����ǻ������Է������������۷�Ӧ����˸÷�Ӧ�Ļ�ѧ����ʽΪ
��Z���Ӻ����Ȼ����ǻ������Է������������۷�Ӧ����˸÷�Ӧ�Ļ�ѧ����ʽΪ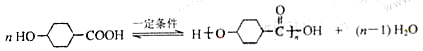 ��
��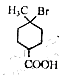 ��֪�������к����Ȼ�����ԭ�ӣ�����C��NaOH����Һ���������·�����ȥ��Ӧ��ͬʱC�У�COOHҲ��NaOH��Ӧ����˸÷�Ӧ�Ļ�ѧ����ʽ��
��֪�������к����Ȼ�����ԭ�ӣ�����C��NaOH����Һ���������·�����ȥ��Ӧ��ͬʱC�У�COOHҲ��NaOH��Ӧ����˸÷�Ӧ�Ļ�ѧ����ʽ��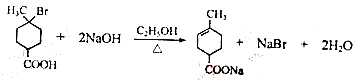 ��
��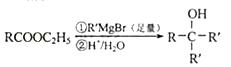 ��G
��G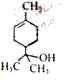 ��֪Ϊ
��֪Ϊ ��
��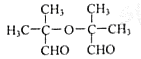 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
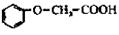 ��������Ӧ�IJ��
��������Ӧ�IJ��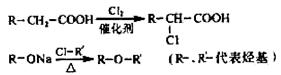
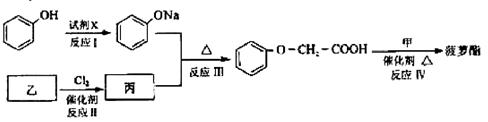
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
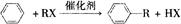 ��RΪ�����XΪ±��ԭ�ӣ���
��RΪ�����XΪ±��ԭ�ӣ���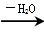 RCH=CH2
RCH=CH2�鿴�𰸺ͽ���>>
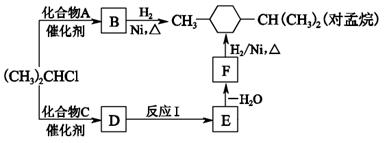
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
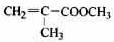 ��
��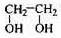 ��
��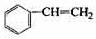 ��
�� 
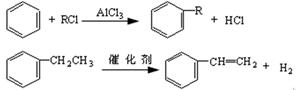
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
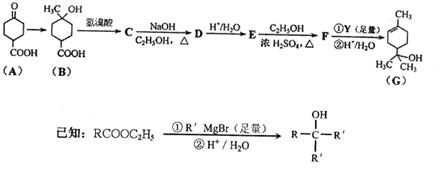
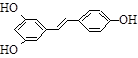 ����������ϩ���ӻ�������п�������������Ԥ����Ѫ�ܼ��������á�ij��������������ºϳ�·�ߣ�
����������ϩ���ӻ�������п�������������Ԥ����Ѫ�ܼ��������á�ij��������������ºϳ�·�ߣ�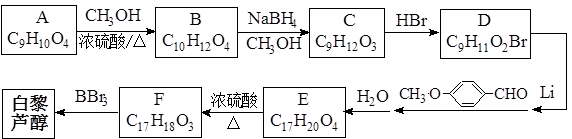

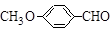 �ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ��_____________________________________________��
�ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ��_____________________________________________���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com