
��15�֣�
����������ѧ��ѧʵ���г��õ�����������ʵ������У�һ�㲻��Ҫ�ò���������
����д��ţ�
����pH��ֽ�ⶨNa2CO3��Һ��pH�� ������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ�� �۽������Ȼ���������Һ�����ˮ���Ʊ������������壻 ��̽��Ba(OH)2 8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ���
8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ���
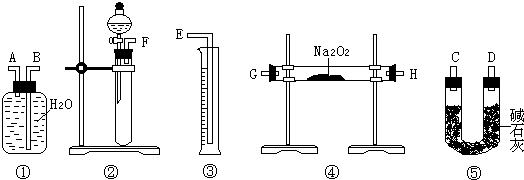
��Ϊ�ⶨij��������Na2O��Na2O2��Ʒ�Ĵ��ȣ�ijС��ͬѧ�ֱ���������·�����
������һ��ȷ������Ʒmg����ˮ��ַ�Ӧ����Һ�����ϡ��ΪVmL������ȡ��V1mL��Һ��װ����ƿ������֪Ũ�ȵ�������еζ�����ȷ����Һ��Ũ�ȣ��ټ������Ʒ��Na2O2�ĺ�����
��1���˷����У�����к͵ζ�ʱӦѡ��ָʾ���� ��
����������ȷ������Ʒmg������Ʒ�������̼��ַ�Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����
��2���÷�����ʵ������У�����������˳���� ���������·���ţ������еĽ�����Ϊ ���A����B����
��3��װ�âݵ������� ��
��4���ڿɹ�ѡ�õķ�Ӧ��ֻ��CaCO3����,6mol/L���������ˮʱ�������һ����IJⶨNa2O2���ȵ�ʵ�鷽�� ��
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com