
��8�֣��Ҵ��ķе���78�棬����ˮ������������ܡ����ѵķе�Ϊ34.6�棬
������ˮ���ڱ���Na2CO3��Һ�м������ܣ����Ѽ���ȼ�ա�ʵ���������ѵ�
��Ӧԭ����

(1) ͼ����������ʵ���������ѵ�װ�ã�ѡװ�� ����ʡ�
(2)��ӦҺ��Ӧ�����ʯ���������� ��
(3)��Ӧ���¶ȼƵ�λ���� ��
(4)��װ�����Ƶõ������п��ܺ��д������Ҵ�����ȥ�Ҵ��ļ������� ��
(5) ����¶�̫�߽��ᷢ����һ�л���Ӧ���˷�Ӧ����ʽΪ
��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2SO4Ũ |
| 140�� |

| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2CH3CH2OH![]() CH3CH2��O��CH2CH3+H2O
CH3CH2��O��CH2CH3+H2O
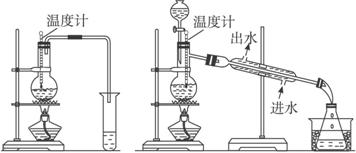
�� ��
(1)ͼ����ͼ��������ʵ���������ѵ�װ�ã�ѡװ��________________����ʣ�������____________________________________________________________________��
(2)��ӦҺ��Ӧ�����ʯ����������_____________________________________________��
(3)��Ӧ���¶ȼƵ�λ����___________________________________________________��
(4)��װ�����Ƶõ������п��ܺ��д��������ʣ���������_____________����ȥ�������ʵļ�������__________________________________________________________________��
(5)����¶�̫�߽������_____________��Ӧ��������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ҵ��ķе���78 �棬����ˮ����������ܣ����ѵķе���34.6 �棬������ˮ���ڱ���Na2CO3��Һ�м������ܣ����Ѽ���ȼ�ա�ʵ���������ѵ�ԭ���ǣ�
2CH3CH2OH![]() CH3CH2��O��CH2CH3+H2O
CH3CH2��O��CH2CH3+H2O
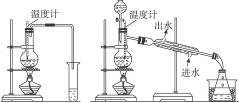
�� ��
��1��ͼ����ͼ��������ʵ���������ѵ�װ�ã�ѡװ��________________����ʣ�������______________________________________________________________________________��
��2����ӦҺ��Ӧ�����ʯ����������_____________________________________________��
��3����Ӧ���¶ȼƵ�λ����___________________________________________________��
��4����װ�����Ƶõ������п��ܺ��д��������ʣ���������_____________����ȥ�������ʵļ�������__________________________________________________________________��
��5������¶�̫�߽������_____________��Ӧ��������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ҵ��ķе���78 �棬����ˮ������������ܡ�����(CH3CH2OCH2CH3)�ķе�Ϊ34.6 �棬������ˮ���ڱ���Na2CO3��Һ�м������ܣ����Ѽ���ȼ�ա�ʵ���������ѵķ�Ӧԭ�����£�
2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
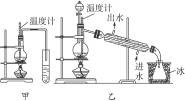
ͼ6-3
(1)ͼ6-3�м���������ʵ���������ѵ�װ�ã�ѡ��װ��__________����ʣ�������______________________________��
(2)��ӦҺ��Ӧ�����ʯ����������________________________________________��
(3)��Ӧ���¶ȼƵ�λ��Ӧ��____________________��
(4)��װ�����Ƶõ������п��ܺ��д������ʣ�����������__________����ȥ�������ʵļ�������____________________��
(5)�����Ӧ�¶�̫�߽��ᷢ��__________��Ӧ��������__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com