
ij��Һ�к��д�����K����Cl����Br��������������Ca2����Mg2����SO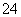 ��ij�о���ѧϰС���������ַ�Һ����ȡ�ϴ������Ȼ��ؾ��弰Һ��(Br2)���������������ͼ��
��ij�о���ѧϰС���������ַ�Һ����ȡ�ϴ������Ȼ��ؾ��弰Һ��(Br2)���������������ͼ��
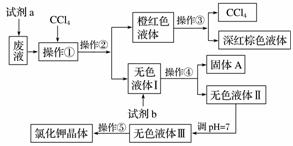
�ɹ��Լ�a���Լ�b(�Լ�b����һ���Լ�)ѡ����Լ�������Na2CO3��Һ������K2CO3��Һ��KOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��Һ��KMnO4��Һ(H��)��ϡ���ᡣ
���������ͼ���ش�������⣺
(1)�Լ�aӦ��ѡ��____________��
(2)�����٢ڢۢܢݵ�������__________(����ĸ)��
A����ȡ�����ˡ���Һ�����ˡ������ᾧ
B����ȡ����Һ�������ˡ������ᾧ
C����Һ����ȡ�����ˡ����ˡ������ᾧ
D����ȡ����Һ����Һ�����ˡ������ᾧ
(3)��ȥ��ɫҺ����е�Ca2����Mg2����SO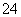 ��ѡ���Լ�b���������Լ������μ�˳��������________________________(�ѧʽ)��
��ѡ���Լ�b���������Լ������μ�˳��������________________________(�ѧʽ)��
(4)����pH��������__________________������������________��
(5)���������õ��Ĵ�������������__________��
�𰸡�(1)H2O2��Һ��(2)B��(3)BaCl2��Һ������K2CO3��Һ��KOH��Һ(��KOH��Һ��BaCl2��Һ������K2CO3��Һ��BaCl2��Һ��KOH��Һ������K2CO3��Һ)��(4)��ȥʣ���OH����CO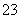 ���μ�ϡ���ᣬ���������ʱ����pH��ֽ�ⶨ��pH��7��(5)������
���μ�ϡ���ᣬ���������ʱ����pH��ֽ�ⶨ��pH��7��(5)������
������(1)�ɼ����CCl4���õ��ijȺ�ɫҺ��֪���Լ�a�ܽ�Br������ΪBr2���Լ�aӦ���������ԣ�����Ӧѡ��H2O2��Һ��
(2)������ͼ֪����ɫҺ����к���K����Cl����Ca2����Mg2����SO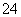 ����ɫҺ�����ֻ����K����Cl�������Լ�b�������dz�ȥCa2����Mg2����SO
����ɫҺ�����ֻ����K����Cl�������Լ�b�������dz�ȥCa2����Mg2����SO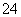 ������������ȡ���������ǽ��������ܵ�����Һ��ֿ�——��Һ���������ǽ��е㲻ͬ�ġ����ܵ���Һ��ֿ�——���������ǽ���Һ������ֿ�——���ˣ��������ǽ�KCl����ˮ��Һ����ȡ����——�����ᾧ��
������������ȡ���������ǽ��������ܵ�����Һ��ֿ�——��Һ���������ǽ��е㲻ͬ�ġ����ܵ���Һ��ֿ�——���������ǽ���Һ������ֿ�——���ˣ��������ǽ�KCl����ˮ��Һ����ȡ����——�����ᾧ��
(3)���ڳ���ʱ�����Լ���������Ҳ������������ʣ���������ȥCa2����ѡ�ñ���K2CO3��Һ������ȥMg2����ѡ��KOH��Һ������ȥSO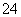 ��ѡ��BaCl2��Һ���������ȥ��ɫ��Һ���е�Ca2����Mg2����SO
��ѡ��BaCl2��Һ���������ȥ��ɫ��Һ���е�Ca2����Mg2����SO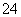 ��ֻҪ����BaCl2��Һ�ڱ���K2CO3��Һ֮ǰ���뼴�ɡ�
��ֻҪ����BaCl2��Һ�ڱ���K2CO3��Һ֮ǰ���뼴�ɡ�
(4)������֪����ɫҺ����л�������������CO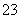 ��OH��������������������ȥʣ���OH����CO
��OH��������������������ȥʣ���OH����CO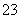 ������pH��7�IJ��������ǵμ����ᣬ���������ʱ����pH��ֽ�ⶨ��pH��7��
������pH��7�IJ��������ǵμ����ᣬ���������ʱ����pH��ֽ�ⶨ��pH��7��
(5)���ڲ������������ᾧ�����Ըò����õ��Ĵ���������������


 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������˳�����K��Na��ǰ�棬K��Na�������Ͼ��кܴ�������ԡ������Ǹ���Na�����ʶ�K�����ʵ�Ԥ�⣬���в���ȷ����(����)
A��K�ڿ����п��Ա������е���������
B��K�������Ҵ�������Ӧ��������
C��K��ˮ�ķ�Ӧ��������ˮ�ķ�Ӧ����
D��KҲ�ɷ���ú���б���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A<B<C<D<E������A��B��C��D��ͬһ���ڵķǽ���Ԫ�ء�Aԭ�ӵ�L��p�������2�����ӣ�C�ǵؿ��ں���(��������)��ߵ�Ԫ�أ�D��Ԫ�����ڱ��ĸ�Ԫ���е縺�����E��ԭ������Ϊ24�����������������ش��������⣺(����ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
(1)A��B��C�ĵ�һ��������С�����˳��Ϊ____________��D��Ԫ�ط�����________��
(2)E��Ԫ�ط�����________����λ�ڵ�________���ڣ����ĺ�������Ų�ʽΪ___�����γɻ�����ʱ��������ϼ�Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һƿ�Ҷ����ͱ������Ļ�����֪���ǵ����������ʾ���ݴ˽��Ҷ����ͱ���������������ѷ�����(����)
| ���� | ����ʽ | �۵�(��) | �е�(��) | �ܶ�(g·cm��3) | �ܽ��� |
| �Ҷ��� | C2H6O2 | ��11.5 | 198 | 1.11 | ������ˮ�;ƾ� |
| ������ | C3H8O3 | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
A.��ȡ�� B���ᾧ��
C����Һ�� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��ӷ�����ȷ����(����)
�ٳ�ȥ��������������ϩ��ͨ��H2���Ӵ�����Ӧ
�ڳ�ȥ��������������������ñ���̼������Һϴ�ӣ���Һ
�۳�ȥNaBr��Һ�е�NaI������ˮ����NaI������CCl4��ȡ��Һ
�ܳ�ȥ�Ҵ��������������������ʯ�ң�����
��ֻ������ɳ�Ĵ��Σ���ͨ���ܽ⡢���ˡ��������ᾧ�ķ����ᴿ
A���٢ڢ� B���ڢܢ� C���ۢܢ� D���ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NH3 ���ӵĿռ乹���������Σ��������������ε�ƽ��ṹ��������ʵ�ij�������� (����)
A��NH3�����Ǽ��Է���
B��������3��N—H���ļ�����ȣ��������
C��NH3������3��N—H���ļ�����ȣ�3�����Ƕ�����107��
D��NH3������3��N—H���ļ�����ȣ�3�����Ƕ�����120��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�����˵���Ƿ���ȷ����ȷ�Ļ����̡�������Ļ�������
(1)�������Ԫ��������Ԫ���γɵĻ�ѧ��(����)
(2)��ȼ��(CH4·8H2O)�м��������ˮ���Ӽ��γ������(����)
(3)�Ҵ����Ӻ�ˮ���Ӽ�ֻ���ڷ��»���(����)
(4)�⻯��ķе�����Ȼ���ķе�����Ϊ�⻯����Ӽ�������(����)
(5)ˮ���Ӽ�ȴ��ڷ��»������ִ������(����)
(6)������з����Ժͱ�����(����)
(7)H2��O2֮��������(����)
(8)H2O2���Ӽ�������(����)
(9)±�ص��ʡ�±���⻯�±��̼����(��CX4)���ۡ��е��������Է������������������(����)
(10)����Ĵ���һ����ʹ���ʵ��ۡ��е�����(����)
(11)���Է����п��ܺ��зǼ��Լ�(����)
(12)H2O��H2S�ȶ�����Ϊˮ���Ӽ�������(����)
�鿴�𰸺ͽ���>>
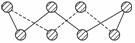
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
S���ʵij�����ʽΪS8���价״�ṹ��ͼ��ʾ��Sԭ�Ӳ��õĹ���ӻ���ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������һ�����Ϳɳ���أ�����ͨ�����ȣ��õ���ܽϳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��
3Zn��2K2FeO4��8H2O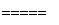 3Zn(OH)2��2Fe(OH)3��4KOH��
3Zn(OH)2��2Fe(OH)3��4KOH��
��ش��������⣺
(1)������صĸ���������________��
(2)�ŵ�ʱ����������________(���������ԭ��)��Ӧ����֪������ӦΪZn��2e����2OH��===Zn(OH)2����������ӦΪ__________________________________________________��
(3)�ŵ�ʱ��________(���������)��������Һ�ļ�����ǿ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com