��2012?������һģ����ҵ�ϵ�ⱥ��ʳ������ȡ���ֻ���ԭ�ϣ����в���ԭ�Ͽ������Ʊ��ྦྷ�裮
��1��ԭ�ϴ����г�������ɳ��Ca
2+��Mg
2+��Fe
3+��SO
42-�����ʣ����뾫�ƺ���ܹ����ʹ�ã�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na
2CO
3����HCl�����ᣩ��BaCl
2����3���Լ����ӵĺ���˳����
�ۢ٢�
�ۢ٢�
������ţ�ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ�Ϊ
��
��
��������ţ����ٱ���Na
2CO
3��Һ �ڱ���K
2CO
3��Һ ��75%�Ҵ��������Ȼ�̼��
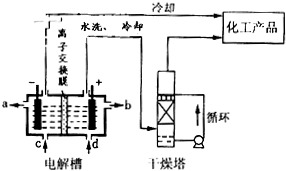
��2����ͼ�����ӽ���Ĥ������������ͨ������������������������ͨ��������ⱥ��ʳ��ˮʾ��ͼ����������������������
����
����
��NaOH��Һ�ij���Ϊ
a
a
������ĸ�������Ʊ���ʳ��ˮ�Ľ���Ϊ
d
d
������ĸ������������Ӧʹ�õ�Һ����
Ũ����
Ũ����
��
�ྦྷ����Ҫ����SiHCl
3��ԭ�����������丱����SiCl
4���ۺ������ܵ��㷺��ע��
��1��SiCl
4���������̿�ڣ�����ά��Ҫԭ����ͬ��������Ϊ������SiCl
4��H
2��O
2��Ӧ�����������֣���ѧ����ʽΪ
��
��2��SiCl
4��ת��ΪSiHCl
3��ѭ��ʹ�ã�һ�������£���20L�����ܱ������еķ�Ӧ��
3SiCl
4��g��+2H
2��g��+Si��s��?4SiHCl
3��g��
��ƽ���H
2��SiHCl
3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H
2ȫ����Դ�����ӽ���Ĥ���ĵ���������������Ĵ�NaCl������Ϊ
0.351
0.351
kg��
��3��ʵ�����Ʊ�H
2��Cl
2ͨ���������з�Ӧ��
Zn+H
2SO
4��ZnSO
4+H
2����MnO
2+4HCl��Ũ��
MnCl
2+Cl
2��+2H
2O
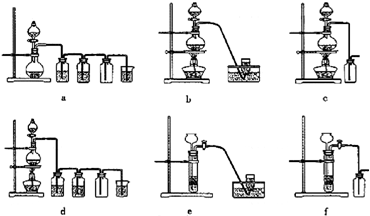
�ݴˣ���������������װ����ѡ���Ʊ����ռ�H
2��װ��
e
e
������ţ����Ʊ����ռ��������Cl
2��װ��
d
d
������ţ���
��ѡ���Ʊ������װ�ã�
��4��������Ĥ���۵�ⱥ��ʳ��ˮ������ȡ�����ƣ�ͬʱ�������������Ƶ�������213.0kg������������
134.4
134.4
m
3����״�����������Կ��ܴ��ڵ�������Ӧ��
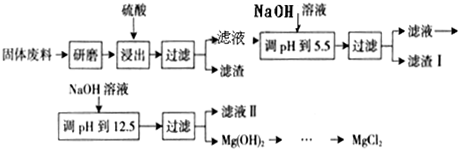
ij����������ɰ�����в����Ĺ�����ϣ���Ҫ����MgCO
3��MgSiO
3��CaMg��CO
3��
2��Al
2O
3��Fe
2O
3�ȣ���������þ�Ĺ����������£�
| ������ |
Fe��OH��3 |
Al��OH��3 |
Mg��OH��2 |
| PH |
3.2 |
5.2 |
12.4 |
����������������������ʽ��ȫ����ʱ��Һ��pH���ϱ�����ش��������⣺
��1���������������У�Ϊ���þ�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ��
�ʵ���߷�Ӧ�¶ȡ����ӽ���ʱ��
�ʵ���߷�Ӧ�¶ȡ����ӽ���ʱ��
��Ҫ��д����������
��2������I����Ҫ�ɷ���
Fe��OH��3��Al��OH��3
Fe��OH��3��Al��OH��3
��
Mg��ClO
3��
2��ũҵ�Ͽ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���
MgCl
2+2NaClO
3��Mg��ClO
3��
2+2NaCl
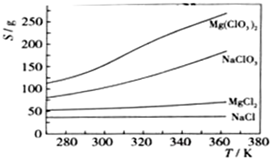
��֪���ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ��ʾ��
��3������Ӧ�ﰴ��ѧ��Ӧ����ʽ�������Ȼ���Ʊ�Mg��ClO
3��
2���������Ʊ�Mg��ClO
3��
2��ԭ��
��ijһ�¶�ʱ��NaCl���ȴﵽ����������Mg��ClO3��2���ܽ�����¶ȱ仯�����NaCl���ܽ�����������ʵ��ܽ����һ���IJ��
��ijһ�¶�ʱ��NaCl���ȴﵽ����������Mg��ClO3��2���ܽ�����¶ȱ仯�����NaCl���ܽ�����������ʵ��ܽ����һ���IJ��
��
��4�����⣨3�������������Ʊ�ʵ�飮����ȴ��������Mg��ClO
3��
2�����У�������NaCl������ԭ���ǣ�
����ǰ����Һ��NaCl�Ѵﱥ�ͣ��������У�NaCl�ܽ�Ȼή�ͣ�������������
����ǰ����Һ��NaCl�Ѵﱥ�ͣ��������У�NaCl�ܽ�Ȼή�ͣ�������������
����ȥ��Ʒ�и����ʵķ����ǣ�
�ؽᾧ
�ؽᾧ
��

 SO3(g)��H=-98 kJ/mol����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�196kJ�����������¶��µ�ƽ�ⳣ��K=_________��
SO3(g)��H=-98 kJ/mol����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�196kJ�����������¶��µ�ƽ�ⳣ��K=_________��  2SO3(g)���ﵽƽ��ı�����������SO2��O2��SO3��ƽ��Ũ�ȶ���ԭ���������_______������ĸ����
2SO3(g)���ﵽƽ��ı�����������SO2��O2��SO3��ƽ��Ũ�ȶ���ԭ���������_______������ĸ���� 
 2SO3(g)��Ӧ��ͼ���У���ȷ����______________��
2SO3(g)��Ӧ��ͼ���У���ȷ����______________�� 
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�





