

·ÖĪö £Ø1£©SO2ŌŚŃĢ³¾µÄ“ß»ÆĻĀŠĪ³ÉĮņĖįŹĒ2SO2+2H2O+O2=2H2SO4£»
£Ø2£©¢Łøł¾Żµē½ā³ŲÖŠŅõŃōĄė×ÓµÄŅĘ¶Æ·½Ļņ£ŗŃōĄė×ÓŅĘĻņŅõ¼«æÉŅŌÅŠ¶Ļ¢ŁĶ¼ÖŠa¼«ŅŖĮ¬½ÓµēŌ“µÄøŗ¼«£¬SO32-ŌŚŃō¼«Ź§Č„µē×Ó±ä³ÉSO42-£¬ĖłŅŌCæŚĮ÷³öµÄĪļÖŹŹĒH2SO4£»
¢ŚSO32-Ź§Č„µē×Ó±»Ńõ»Æ³ÉSO42-£»
¢Ūµē½ā¹ż³ĢÖŠŅõ¼«Ēų·ÅĒāÉś¼ī£¬Ė®±äĪŖĒāŃõ»ÆÄĘ£¬Ņõ¼«Ēų±ä»ÆµÄÖŹĮæµČÓŚĒāŃõ»ÆÄʵÄÖŹĮæ¼õČ„ĻūŗÄĖ®µÄÖŹĮ棬øł¾Żµē×ÓŹŲŗć¼ĘĖć£»
£Ø3£©¢ŁČÜŅŗÖŠS032-Ė®½ā£ŗSO32-+H2O?HSO3-+OH-£¬ĘĘ»µĖ®µÄµēĄėĘ½ŗā£¬µ¼ÖĀČÜŅŗĻŌ¼īŠŌ£¬øł¾ŻµēŗÉŹŲŗć·ÖĪö£»
¢Ś³£ĪĀĻĀ£¬ŃĒĮņĖįĒāÄĘ³ŹĖįŠŌ£¬ĖµĆ÷ŃĒĮņĖįĒāøłĄė×ӵĵēĄė³Ģ¶Č“óÓŚĖ®½ā³Ģ¶Č£¬øł¾ŻĪļĮĻŹŲŗćµĆc£ØNa+£©=c£ØH2SO3£©+c£ØHSO3-£©+c£ØSO32-£©¢Ł£¬øł¾ŻµēŗÉŹŲŗćµĆc£ØNa+£©+c£ØH+£©=c£ØOH-£©+c£ØHSO3-£©+2c£ØSO32-£©¢Ś£¬Į½Ź½¼ĘĖćµĆµ½£®
½ā“š ½ā£ŗ£Ø1£©SO2ŌŚŃĢ³¾µÄ“ß»ÆĻĀŠĪ³ÉĮņĖįŹĒ2SO2+2H2O+O2=2H2SO4£¬
¹Ź“š°øĪŖ£ŗ2SO2+2H2O+O2=2H2SO4£»
£Ø2£©¢Łøł¾Żµē½ā³ŲÖŠŅõŃōĄė×ÓµÄŅĘ¶Æ·½Ļņ£ŗŃōĄė×ÓŅĘĻņŅõ¼«æÉŅŌÅŠ¶Ļ¢ŁĶ¼ÖŠa¼«ŅŖĮ¬½ÓµēŌ“µÄøŗ¼«£¬SO32-ŌŚŃō¼«Ź§Č„µē×Ó±ä³ÉSO42-£¬ĖłŅŌCæŚĮ÷³öµÄĪļÖŹŹĒH2SO4£¬
¹Ź“š°øĪŖ£ŗøŗ£»ĮņĖį£»
¢ŚSO32-Ź§Č„µē×Ó±»Ńõ»Æ³ÉSO42-£¬µē¼«·“Ó¦Ź½ĪŖ£ŗSO32--2e-+H2O=SO42-+2H+£¬
¹Ź“š°øĪŖ£ŗSO32--2e-+H2O=SO42-+2H+£»
¢Ūµē½ā¹ż³ĢÖŠŅõ¼«Ēų·ÅĒāÉś¼ī£¬Ė®±äĪŖĒāŃõ»ÆÄĘ£¬Ņõ¼«Ēų±ä»ÆµÄÖŹĮæµČÓŚĒāŃõ»ÆÄʵÄÖŹĮæ¼õČ„ĻūŗÄĖ®µÄÖŹĮ棻
øł¾Żµē×ÓŹŲŗć£ŗ
2e-”«Na2SO3£¬”«2H2O”«2NaOH”«Ņõ¼«Ēų±ä»ÆµÄÖŹĮæ
126g 36g 80g 44g
6.3g x
x=2.2g
ČōĻūŗÄ 6.3gNa2SO3£¬ŌņŅõ¼«Ēų±ä»ÆµÄÖŹĮæĪŖ2.2g£¬
¹Ź“š°øĪŖ£ŗ2.2£»
£Ø3£©¢ŁČÜŅŗÖŠS032-Ė®½ā£ŗSO32-+H2O?HSO3-+OH-£¬ĘĘ»µĖ®µÄµēĄėĘ½ŗā£¬µ¼ÖĀČÜŅŗĻŌ¼īŠŌ£¬øł¾ŻµēŗÉŹŲŗć£¬ČÜŅŗÖŠc£ØH+£©+c£ØNa+£©=2c£ØSO32-£©+c£ØHSO3-£©+c£ØOH-£©£¬¹Źc£ØNa+£©£¼2c£ØSO32-£©+c£ØHSO3-£©+c£ØOH-£©£¬
¹Ź“š°øĪŖ£ŗSO32-+H2O?HSO3-+OH-£»£¼£»
¢Ś³£ĪĀĻĀ£¬ŃĒĮņĖįĒāÄĘ³ŹĖįŠŌ£¬ĖµĆ÷ŃĒĮņĖįĒāøłĄė×ӵĵēĄė³Ģ¶Č“óÓŚĖ®½ā³Ģ¶Č£¬øł¾ŻĪļĮĻŹŲŗćµĆc£ØNa+£©=c£ØH2SO3£©+c£ØHSO3-£©+c£ØSO32-£©¢Ł£¬øł¾ŻµēŗÉŹŲŗćµĆc£ØNa+£©+c£ØH+£©=c£ØOH-£©+c£ØHSO3-£©+2c£ØSO32-£©¢Ś£¬½«·½³ĢŹ½¢Ł“śČė¢ŚµĆ£¬c£ØH2SO3£©+c£ØH+£©=c£ØOH-£©+c£ØSO32-£©£¬ĖłŅŌc£ØSO32-£©-c£ØH2SO3£©=c£ØH+£©-c£ØOH-£©=£Ø10-6-10-8£©mol•L-1=9.9”Į10-7mol•L-1£¬
¹Ź“š°øĪŖ£ŗ9.9”Į10-7£®
µćĘĄ ±¾Ģāæ¼²éĮĖµē½ā³ŲŌĄķŗĶµē¼«·“Ó¦ŹéŠ“”¢µē½āÖŹČÜŅŗÖŠµēŗÉŹŲŗć”¢ĪļĮĻŹŲŗćµČÖŖŹ¶µćµÄĄķ½āÓ¦ÓĆ£¬ÕĘĪÕ»ł“”ŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£¬ÓŠĄūÓŚĢįøßѧɜĮé»īŌĖÓĆĖłŃ§ÖŖŹ¶½ā¾öŹµ¼ŹĪŹĢāµÄÄÜĮ¦£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | Ķ¼1ÖŠµē½āÖŹČÜŅŗµÄpHŌö“ó | |
| B£® | Ķ¼2ÖŠµē½āAlCl3ČÜŅŗµÄ×Ü·“Ó¦ĪŖ2Cl-+2H2O$\frac{\underline{\;µē½ā\;}}{\;}$Cl2”ü+H2”ü+2OH- | |
| C£® | A“¦ĶØČėµÄĘųĢåĪŖCH4£¬µē¼«·“Ó¦Ź½ĪŖCH4+10OH--8e-ØTCO32-+7H2O | |
| D£® | µē½ā³ŲÖŠCl-Ļņx¼«ŅĘ¶Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | KCl | B£® | NaOH | C£® | CCl4 | D£® | H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | CO”¢SO2”¢SO3¾łŹĒĖįŠŌŃõ»ÆĪļ | |
| B£® | Ķ¼Ź¾×Ŗ»Æ·“Ó¦¾łĪŖŃõ»Æ»¹Ō·“Ó¦ | |
| C£® | ¹¤ŅµÉĻÓĆĀČĘųŗĶŹÆ»ŅČéÖĘČ”ĘÆ°×·ŪµÄ·“Ó¦ÖŠŌ×ÓĄūÓĆĀŹĪŖ100% | |
| D£® | ŃĻ½ūÓĆ¹¤Ņµ¾Ę¾«¹“¶ŅŅūÓĆ¾ĘŹĒŅņĪŖ¹¤Ņµ¾Ę¾«ÖŠŗ¬ÓŠÓŠ¶¾µÄ¼×“¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓƱ„ŗĶNa2CO3ČÜŅŗ³żČ„SO2ĘųĢåÖŠŗ¬ÓŠµÄÉŁĮæCO2 | |
| B£® | ÓĆ¼ÓČȵķ½·ØĢįČ”NH4Cl¹ĢĢåÖŠ»ģÓŠµÄÉŁĮæµ„ÖŹµā | |
| C£® | ÓĆ²£Į§°ō½Į°čĀ©¶·ÖŠµÄŅŗĢåŅŌ¼Óæģ¹żĀĖµÄĖŁ¶Č | |
| D£® | ÓĆ“×ŗĶKIµķ·ŪČÜŅŗ¼ģŃéŹ³ÓĆ¼ÓµāŃĪÖŠŗ¬ÓŠµÄµāŌŖĖŲ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŖĖŲQŹĒ¶ĢÖÜĘŚ·Ē½šŹōŠŌ×īĒæµÄŌŖĖŲ | |
| B£® | Y”¢ZŠĪ³ÉµÄ»ÆŗĻĪļÖŠÖ»ÓŠŅ»ÖÖŹōÓŚĖįŠŌŃõ»ÆĪļ | |
| C£® | Y”¢Z”¢Q·Ö±šÓėWŠĪ³ÉµÄ»ÆŗĻĪļÖŠ£¬ZŠĪ³ÉµÄ»ÆŗĻĪļµÄ·Šµć×īµĶ | |
| D£® | X·Ö±šÓėY”¢Z”¢WŠĪ³ÉµÄ»ÆŗĻĪļÖŠæÉÄܼČŗ¬Ąė×Ó¼üÓÖŗ¬¹²¼Ū¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH3CH2CH2CH2CH2Cl | B£® | 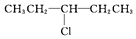 | ||
| C£® | 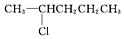 | D£® | 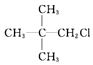 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com