
��ʵ������ģ����N2��H2�ϳɰ�������δ�������N2��H2�������V(N2)��V(H2)��1��3��ֻ�Ϻ���ͼ��ʾ��װ�ý����й�ʵ�飮
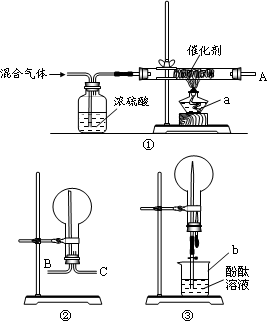
��ش��������⣺
(1)ʵ������a��b�����Ʒֱ���________��________��
(2)װ�â���Ũ�����������________��
(3)�����װ�â��ռ���Ӧ���ɵ����壬�ܿ�AӦ��װ���е�________(�B����C��)�ڣ��ռ���������ɷ���________��
(4)�ռ������������װ�â۽���ʵ�飬����ƿ�е�Һ������������Һ��________ɫ��
(5)ʵ�����ʱ���������Һ�����Ϊ��ƿ�ݻ���![]() �����ںϳɰ�������N2��ת����Ϊ________��(
�����ںϳɰ�������N2��ת����Ϊ________��(![]() )
)
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?��Զģ�⣩�¹��˹�����1909�귢���ĺϳɰ���Ӧԭ��Ϊ��N2��g��+3H2��g��?2NH3��g�� ��֪298Kʱ����H=-92.4kJ?mol-1�Իش��������⣺
��2009?��Զģ�⣩�¹��˹�����1909�귢���ĺϳɰ���Ӧԭ��Ϊ��N2��g��+3H2��g��?2NH3��g�� ��֪298Kʱ����H=-92.4kJ?mol-1�Իش��������⣺| 0.082 |
| 0.06��0.183 |
| 0.082 |
| 0.06��0.183 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������һ����Ҫ�Ļ���ԭ�ϣ������Ź�ҵ�����̵ļӿ죬����Ҳ��ɾ�����һ����Ҫ����Ⱦ�����壮
������һ����Ҫ�Ļ���ԭ�ϣ������Ź�ҵ�����̵ļӿ죬����Ҳ��ɾ�����һ����Ҫ����Ⱦ�����壮
| ||
| ||
| C2(NH3)?C(CO2) |
| C(H2O) |
| C2(NH3)?C(CO2) |
| C(H2O) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�±���Ԫ�����ڱ���һ���֣�
(1)����Ԫ�آ���⻯��Ļ�ѧʽΪ �����⻯��Ļ�ԭ�Ա�Ԫ�آ���⻯��Ļ�ԭ�� ����ǿ������
(2)ijԪ��ԭ�ӵĺ�����Ӳ���L���������K���������3�����Ԫ�ص�Ԫ�ط����� ���䵥�ʵĵ���ʽΪ ��
(3)��֪ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���Ԫ�آ���Ԫ�آ���������������Ƶ����ʡ�д��Ԫ�آ۵�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
��
���������Ԫ�آߵ��������ƵIJ�ͬ��Ԫ���� ����Ԫ�ط��ţ�
(4)Ԫ�آݵ��⻯��������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ���α�����ܵĺϳɰ������й��������ǵ¹��˹�����1905�귢���ģ���ϳ�ԭ��Ϊ��N2(g)+3H2(g)![]() 2NH3(g) ��H= ��92.4kJ/mol������˻����1918��ŵ������ѧ�����Իش��������⣺
2NH3(g) ��H= ��92.4kJ/mol������˻����1918��ŵ������ѧ�����Իش��������⣺
�ٺϳɰ���ҵ�в�ȡ�����д�ʩ������������ԭ�����͵��� ������ţ�
A�����ýϸ�ѹǿ��20Mpa~50Mpa��
B������500��ĸ���
C��������ý������
D�������ɵİ�Һ������ʱ����ϵ�з��������N2��H2ѭ�����ϳ����в�����N2��H2
����ͼ��ʵ����ģ�ҵ���ϳɰ��ļ���װ�á����������а������ɵķ��� ��
����298Kʱ����10 mol N2��30 mol H2����ϳ����У�Ϊ�ηų�������С��924kJ��
��
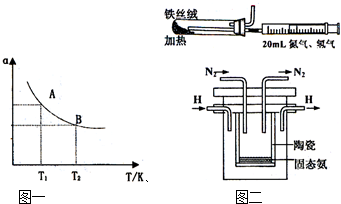
��1998��ϣ������˹��´�ѧ��Marmellos��Stoukides����һ�ֺϳɰ����·������ڳ�ѹ�£���H2����Heϡ�͵�N2�ֱ�ͨ��һ���ȵ�570��ģ����ȣ��棭�ƣ����ѿ����մɣ�SCY��Ϊ�������ʵĵ�����ת��Ϊ������SCY�մ��ܴ���H+����H2ת���ʴﵽ78������ʵ��װ����ͼ��ʾ�������ĵ缫��Ӧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������»���ѧ������ѧ�ڵ��Ĵ��¿���ѧ�Ծ� ���ͣ������
�±���Ԫ�����ڱ���һ���֣�

(1)����Ԫ�آ���⻯��Ļ�ѧʽΪ �����⻯��Ļ�ԭ�Ա�Ԫ�آ���⻯��Ļ�ԭ�� ����ǿ������
(2)ijԪ��ԭ�ӵĺ�����Ӳ���L���������K���������3�����Ԫ�ص�Ԫ�ط����� ���䵥�ʵĵ���ʽΪ ��
(3)��֪ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���Ԫ�آ���Ԫ�آ���������������Ƶ����ʡ�д��Ԫ�آ۵�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
��
���������Ԫ�آߵ��������ƵIJ�ͬ��Ԫ���� ����Ԫ�ط��ţ�
(4)Ԫ�آݵ��⻯��������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ���α�����ܵĺϳɰ������й��������ǵ¹��˹�����1905�귢���ģ���ϳ�ԭ��Ϊ��N2(g)+3H2(g) 2NH3(g) ��H= ��92.4kJ/mol������˻����1918��ŵ������ѧ�����Իش��������⣺
2NH3(g) ��H= ��92.4kJ/mol������˻����1918��ŵ������ѧ�����Իش��������⣺
�ٺϳɰ���ҵ�в�ȡ�����д�ʩ������������ԭ�����͵��� ������ţ�
A�����ýϸ�ѹǿ��20Mpa~50Mpa��
B������500��ĸ���
C��������ý������
D�������ɵİ�Һ������ʱ����ϵ�з��������N2��H2ѭ�����ϳ����в�����N2��H2
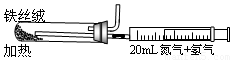
����ͼ��ʵ����ģ�ҵ���ϳɰ��ļ���װ�á����������а������ɵķ��� ��
����298Kʱ����10 mol N2��30 mol H2����ϳ����У�Ϊ�ηų�������С��924kJ��
��
��1998��ϣ������˹��´�ѧ��Marmellos��Stoukides����һ�ֺϳɰ����·������ڳ�ѹ�£���H2����Heϡ�͵�N2�ֱ�ͨ��һ���ȵ�570��ģ����ȣ��棭�ƣ����ѿ����մɣ�SCY��Ϊ�������ʵĵ�����ת��Ϊ������SCY�մ��ܴ���H+����H2ת���ʴﵽ78������ʵ��װ����ͼ��ʾ�������ĵ缫��Ӧʽ ��
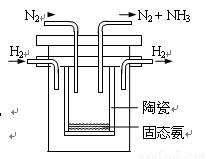
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com