
���� ��1���ٸ�����������������ʣ���Һ�в��ֵ���������ӣ��������������Һ���ܹ�����ˮ�⣬ǿ��������ˮ����ʾ���ԵȽ����жϣ�Ҳ����ͨ����Һ��ϡ�ͣ�������ҺpH�仯�ж�HA�������
�ڸ�����Һ�еĵ���غ�������غ������
�۸��ݷ�Ӧ����ʽ�жϳ�����ǿ��������Խ�������ε�ˮ��̶�Խ��PH��ͬʱ��Ũ��ԽС��
��2����c�� HA��=c��NaOH��=0.1mol/L����û�Ϻ���Һ��pH=7��˵��HAΪǿ�ᣬ
����HAŨ��Ϊc�����ΪV�����У�$\frac{cV-0.1V}{2V}$=$\frac{cV}{10V}$���Դ˽��м��㣻
�ڸ���c���ᣩ��V���ᣩ=V�����c�������������������c���ᣩ��Ӱ�죬�Դ��ж�Ũ�ȵ���
��3������Һ�����ԣ�����c��H+����c��OH-����������Һ�����Է�����
�ڼ��㷴Ӧ����Һ��c��H+��������c��H+����c��OH-��=10-12���㣮
��� �⣺��1����A�����0.1mol/L��HA��Һ��pH��1��˵����Һ��������Ũ��С��0.1mol/L��HA����Һ�в��ֵ���������ӣ�HA�������ᣬ��A��ȷ��
B�����NaA��Һ��pH��7��˵��A-��ˮ�⣬��HA�������ᣬ��B��ȷ��
C��pH=l��HA��Һ�����ᣬϡ��100���������pH�仯��˵��HA�д��ڵ���ƽ�⣬��HA�������ᣬ��C��ȷ��
D��������п�ֱ�����ͬpH����ͬ����������HA��Һ��Ӧ������������һ���࣬˵��HA�е����������������HClһ���࣬��˵��HAΪǿ�ᣬ��D����
�ʴ�Ϊ��D��
��A����֪�����Һ�д��ڵ���غ�Ϊ��c��A-��+c��OH-��=c��Na+��+c��H+������c��A-����c��Na+������c��OH-����c��H+������A����
B����֪�����Һ�д��ڵ���غ�Ϊ��c��A-��+c��OH-��=c��Na+��+c��H+������B��ȷ��
C�������Һ�д��������غ㣬��Ϻ�Ũ�ȱ�Ϊԭ����һ�룬��c��HA��+c��A-��=0.05mol/L����C����
D���������غ�ã�c��HA��+c��A-��=c��Na+��������غ�Ϊ��c��A-��+c��OH-��=c��Na+��+c��H+����������ʽ����ģ�c�� HA��+c�� H+��=c��OH-������D��ȷ��
�ʴ�Ϊ��BD��
����HA+B2-��������=A-+HB-��H2B��������+2C-=B2-+2HC��HA+C-=A-+HC�������ԣ�H2B��HA��HB-��HC������Խ�������ε�ˮ��̶�Խ��pH��ͬʱ��Ũ��ԽС���������ʵ���Ũ���ɴ�С��˳��Ϊ���ۢ٢ڢܣ�
�ʴ�Ϊ���ۢ٢ڢܣ�
��2����c�� HA��=c��NaOH��=0.1mol/L����û�Ϻ���Һ��pH=7��˵��HAΪǿ�ᣬ
����HAŨ��Ϊc�����ΪV�����У�$\frac{cV-0.1V}{2V}$=$\frac{cV}{10V}$����c=0.125��
�ʴ�Ϊ��0.125��
��A��������ˮϴ����ƿ���ô���HA��Һ������ϴ����V���ƫ������HAŨ��ƫ��A��ȷ��
B���ζ�ǰ���ֵζ��ܵļ��첿�������ݣ��ζ�����ʧ����V���ƫ������HAŨ��ƫ��B��ȷ��
C��װNaOH�ļ�ʽ�ζ���δ�ñ���NaOH��Һ��ϴ����V���ƫ������HAŨ��ƫ��C��ȷ��
D���ζ�ǰ���ӣ��ζ����Ӷ������ᵼ����V���ƫС������HAŨ��ƫС����D����
�ʴ�Ϊ��ABC��
��3������Һ�����ԣ�����c��H+����c��OH-����������Һ�ʵ����ԣ�����c��Na+��+c��H+��=c��CH3COO-��+c��OH-��������c��CH3COO-����c��Na+������Ȼ��Ӧ��ʣ��Ĵ���������ӵ����ʵ�����ȣ�������Ϊ������ʣ����ֵ��룬����c��Na+����c��H+��������c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
����HAΪǿ�ᣬ��Ӧ������������У�c��H+��=$\frac{0.04mol/L��V-0.02mol/L��V}{2V}$=0.01mol/L��
99��ʱ��Kw=10-12����c��H+����c��OH-��=10-12��
c��OH-��=10-10����ˮ�������c��H+����ȣ�
�ʴ�Ϊ��10-10��
���� ���⿼������϶����жϼ���ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ��漰����ϵļ��㡢�����ˮ���Լ�������ʵĵ��룬�����ע��������Һ�������غ㡢�����غ��Լ�����غ��֪ʶ��Ӧ�÷�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 283.5kJ/mol | B�� | 172.5kJ/mol | C�� | -172.5kJ/mol | D�� | -504 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
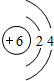 ��DԪ����Ԫ�����ڱ��е�λ��Ϊ�������ڵڢ�A�壮
��DԪ����Ԫ�����ڱ��е�λ��Ϊ�������ڵڢ�A�壮 ��C��D��Ԫ���γɵĻ����ﻯѧ������Ϊ���Ӽ���
��C��D��Ԫ���γɵĻ����ﻯѧ������Ϊ���Ӽ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 111g•mol-1 | B�� | 100g•mol-1 | C�� | 27.5g•mol-1 | D�� | 55g•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | WX2������Wԭ�Ӳ�ȡsp3�ӻ� | |
| B�� | WX2���Լ��Լ���ϳɵķǼ��Է��� | |
| C�� | WX2��YX2�еĻ�ѧ���;������Ͷ���ͬ | |
| D�� | ԭ�Ӱ뾶��С˳��Ϊ��X��W��Y��Z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com