
������������A��B��C��D��E�����ǵ������ӿ�����Na����NH4����Cu2����Ba2����Al3���� Ag����Fe3���������ӿ�����Cl����NO3����SO42����CO32������֪��
Ag����Fe3���������ӿ�����Cl����NO3����SO42����CO32������֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⡣
(1)�������У�һ��û�е���������________��������������ͬ�������εĻ�ѧʽ��________��
(2)D�Ļ�ѧʽΪ________��D��Һ�Լ��Ե�ԭ����(�����ӷ���ʽ��ʾ)_______________________________________________________________
________________________________________________________________��
(3)A��C����Һ��Ӧ�����ӷ���ʽ��________��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��________________________________________________________ _____��
_____��
(4)��Ҫ����B�������������ӣ���ȷ��ʵ�鷽����______________________
_________________________________________________________________��
��������������Һû����ɫ������û��Cu2����Fe3��������ʵ�������֪��A��B��C��D��E�ֱ�ΪBaCl2��(NH4)2SO4��AgNO3��Na2CO3��Al2(SO4)3������NH4�������õķ����ǽ������ŨNaOH��Һ���ȣ����Ƿ��ܹ�����ʹʪ��ĺ�ɫʯ����ֽ���������塣
�𰸡�(1)Cu2����Fe3����(NH4)2SO4��Al2(SO4)3
(2)Na2CO3��CO32����H2O  HCO3����OH��
HCO3����OH��
(3)Ag����Cl��===AgCl��
Al3����3NH3��H2O===Al(OH)3����3NH4��
(4)ȡ����B���Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲��Ƿ����ɫ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������������ʴ��ÿ����ʴ����ʧ�ĸ���ռ���������������ķ�֮һ��
(1)������ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��ӦʽΪ___________________��
(2)Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ���ͼ����ʾ�ķ��������к�������բ ���ϵĹ������R���Բ���________(��д��ĸ���)��
A��ͭ B���� C��п D��ʯī
(3)ͼ����ʾ�ķ���Ҳ���Խ�����բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ��________����
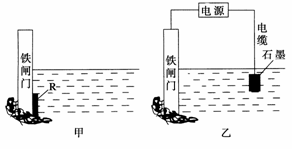
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪HCO3�D+AlO2�D +H2O=CO32�D +Al(OH)3������������KHCO3��Һ���ϵ��뺬�����ʵ�����KOH��Ba(OH)2��KAlO2�Ļ����Һ�У����ɳ��������ʵ���������KHCO3��Һ����Ĺ�ϵ�ɱ�ʾΪ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ݱ�������һ�ֽ�Thibacillus Ferroxidans��ϸ�������������µ�������Һ�У��ܽ���ͭ��(CuFeS2)�����������Σ������ķ�ӦΪ��
4CuFeS2��2H2SO4��17O2===4CuSO4��2Fe2(SO4)3��2H2O
(1)CuFeS2��Fe�Ļ��ϼ�Ϊ��2��������Ӧ�б�������Ԫ����________��
(2)��ҵ����������������Ӧ�����Һ�����������̿��Ʊ�����(CuSO4��5H2O)��
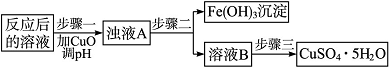
�ٷ������б���(����Ksp����Ӧ������������ij����ܽ�ƽ�ⳣ��)��
| Ksp | �������↑ʼ ����ʱ��pH | ����������� ��ȫʱ��pH | |
| Fe3�� | 2.6��10��39 | 1.9 | 3.2 |
| Cu2�� | 2.2��10��20 | 4.7 | 6.7 |
����һӦ������Һ��pH��Χ��__________�������ó����ܽ�ƽ����й����۽��ͼ���CuO�ܳ�ȥCuSO4��Һ��Fe3����ԭ��________________________________________________
________________________________________________________________________��
�ڲ������еľ������������_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����и�����������Һ�У�һ���ܴ���������������� (����)��
A����ɫ��Һ��Ca2����H����Cl����HSO3��
B����ʹpH��ֽ�ʺ�ɫ����Һ��Na����NH4����I����NO3��
C��FeCl2��Һ��K ����Na����SO42����AlO2��
����Na����SO42����AlO2��
D.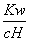 ��0.1 mol��L��1����Һ��Na����K����SiO32����NO3��
��0.1 mol��L��1����Һ��Na����K����SiO32����NO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ը����л���Ľṹ�����ü���ʽ��ʾ�����л���CH2=CHCHO���Լ�д�� ���������ʽΪ
���������ʽΪ �����ʻ�Ϊͬ���칹�����(����)
�����ʻ�Ϊͬ���칹�����(����)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���Ľṹ��ʽ����ͼ��
����л���ɷ����ķ�Ӧ�����У�
��ȡ�� �ڼӳ� ����ȥ ������ ��ˮ������� ���к�( )
A.�٢ڢۢݢ� B.�ڢۢܢݢ� C.�٢ڢۢܢݢ� D.�٢ڢۢܢݢޢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
100 mL 2 mol/L H2SO4 �����Zn�۷�Ӧ����һ���¶��£�Ϊ�˼ӿ췴Ӧ���ʣ����ֲ�Ӱ���������������������Բ�ȡ�Ĵ�ʩ�ǣ� ��
A������̼���ƹ��� B������18 mol/L��ŨH2SO4
C��������������ͭ��Һ D�������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮϡ��0.1mol/L��ˮʱ����Һ������ˮ�������Ӷ���С���ǣ� ��
A��[OH-]/[NH3��H2O] B.[NH3��H2O]/[OH-]
C��[H+]��[OH-]�ij˻� D.OH-�����ʵ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com