


 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
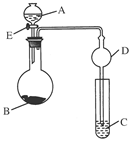
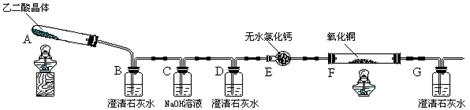
 CaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1~100nm֮�䣩����ͼ��ʾA~EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⡣
CaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1~100nm֮�䣩����ͼ��ʾA~EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⡣
| A����ʯ�� | B����ʯ�� | C����ˮ�Ȼ��� | D����ˮ����ͭ E���ռ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| �е�/�� | 57.7 | 12.8 | �� | 315 | �� |
| �۵�/�� | ��70.0 | ��107.2 | �� | �� | �� |
| �����¶�/�� | �� | �� | 180 | 300 | 162 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���ô��ű�ʾ)��
���ô��ű�ʾ)��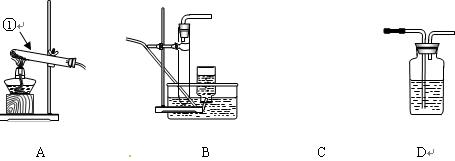
 ���ƶ�����̼ʱ��Ӧѡ��
���ƶ�����̼ʱ��Ӧѡ�� �ķ���װ����__ __�����������̼�����ѡ��Dװ
�ķ���װ����__ __�����������̼�����ѡ��Dװ �ã���װ����ʢ�ŵ��Լ�һ����__ __��
�ã���װ����ʢ�ŵ��Լ�һ����__ __���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
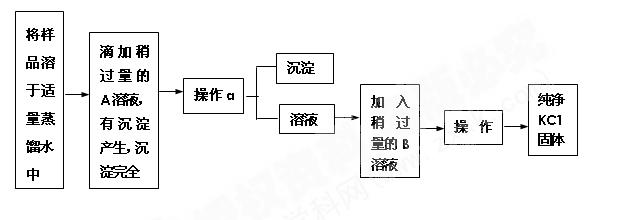
�鿴�𰸺ͽ���>>
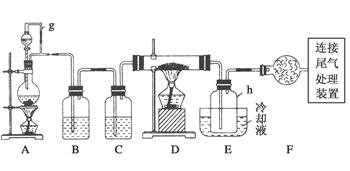
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | �ζ�ǰ | ��һ���յ� | �ڶ����յ� | �������յ� |
| �ζ���Һ��̶� | 0.00mL | 16.02mL | 16.00mL | 16.01mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com