

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”ļ”ļ”ļ
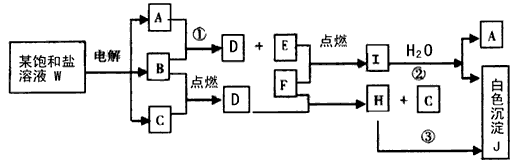
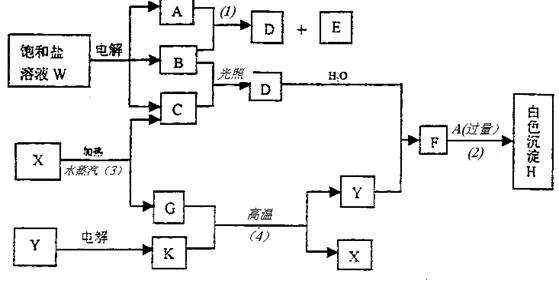
±„ŗĶŃĪČÜŅŗWµÄµē½ā²śĪļ·¢ÉśĻĀĮŠĻµĮŠ·“Ó¦”£Ķ¼ÖŠµÄĆæŅ»·½øń±ķŹ¾ÓŠ¹ŲµÄŅ»ÖÖÖ÷ŅŖ·“Ó¦Īļ»ņÉś³ÉĪļ£Ø·“Ó¦ÖŠ¼ÓČė»ņÉś³ÉµÄĖ®ŅŌ¼°Éś³ÉµÄĘäĖü²śĪļŅŃĀŌČ„£©£¬ĘäÖŠA”¢B”¢C”¢D”¢EŌŚ³£ĪĀĻĀ¾łĪŖøßÖŠ»Æѧ֊³£¼ūĘųĢ¬ĪļÖŹ£¬XŗĶKŹĒ֊ѧ³£¼ūµÄ½šŹōµ„ÖŹ£¬Ńõ»ÆĪļYŹĒŅ»ÖÖ±Č½ĻŗƵÄÄĶ»š²ÄĮĻ”£HæÉČÜÓŚĒæĖįĒæ¼ī”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁWµÄĆū³ĘŹĒ £¬GµÄĖ×ĆūŹĒ ”£
¢ŚA·Ö×ÓµÄæռ乹ŠĶŹĒ ”£
¢Ū·“Ó¦£Ø3£©µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
·“Ó¦£Ø4£©µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
¢Ü±„ŗĶŃĪČÜŅŗWµē½āµÄĄė×Ó·½³ĢŹ½ŹĒ ”£
¢ŻŅŃÖŖCµÄČ¼ÉÕČČĪŖ285.8kJ/mol”£ŹŌŠ“³öCĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬ĪļÖŹµÄČČ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com