
·ÖĪö ŅŃÖŖ£ŗŹŅĪĀĻĀ£¬±„ŗĶĒāŃõ»ÆøĘČÜŅŗÅضČŌ¼ĪŖ0.00165 g/mL£¬Ōņ1LČÜŅŗÖŠ×ī¶ąŗ¬ÓŠ1.65gøĘĄė×Ó£¬×ī“óĪļÖŹµÄĮæĪŖ£ŗ$\frac{1.65g}{40g/mol}$=0.04125mol£¬ŌņĮłÖÖ»ÆŗĻĪļÖŠ²»æÉÄÜŗ¬ÓŠĒāŃõ»ÆøĘ£¬
¢Ł²āµĆČÜŅŗA”¢C”¢E³Ź¼īŠŌ£¬ČżÖÖČÜŅŗĪŖ¼īŅŗ»ņĖ®½ā³Ź¼īŠŌµÄČÜŅŗ£¬ĒŅ¼īŠŌĪŖA£¾E£¾C£¬ŌņAĪŖ¼ī£¬ČÜŅŗÖŠŗ¬ÓŠ“óĮæµÄOH-Ąė×Ó£¬OH-Ąė×ÓÓėAg+£¬Ca2+£¬Fe2+£¬Al3+µČĄė×Ó²»ÄÜ“óĮæ¹²“ę£¬¹ŹAÖ»ÄÜĪŖBa£ØOH£©2£¬øł¾ŻŌ½ČõŌ½Ė®½ā£¬EÓ¦ĪŖĢ¼ĖįŃĪ£¬øł¾ŻĄė×Ó¹²“ę£¬Ö»ÄÜĪŖK2CO3£¬CĪŖ“×ĖįŃĪ£»
¢ŚĻņBČÜŅŗÖŠµĪ¼ÓNa2SČÜŅŗ£¬³öĻÖÄŃČÜÓŚĒæĖįĒŅŅõŃōĄė×ÓøöŹż±ČĪŖ1£ŗ2µÄŗŚÉ«³Įµķ£¬øĆŗŚÉ«³ĮµķĪŖĮņ»ÆŅų£¬ŌņBĪŖĻõĖįŅųČÜŅŗ£»
¢ŪĻņDČÜŅŗÖŠµĪ¼ÓBa£ØNO3£©2ČÜŅŗ£¬ĪŽĆ÷ĻŌĻÖĻó£¬ĖµĆ÷BÖŠ²»ŗ¬SO42-Ąė×Ó£»
¢ÜĻņFČÜŅŗÖŠµĪ¼Ó°±Ė®£¬Éś³É°×É«Šõד³Įµķ£¬³ĮµķŃøĖŁ±ä³É»ŅĀĢÉ«£¬×īŗó±ä³ÉŗģŗÖÉ«£¬ĖµĆ÷FÖŠŗ¬ÓŠFe2+Ąė×Ó£»
×ŪÉĻ·ÖĪöæÉÖŖ£¬AĪŖBa£ØOH£©2£¬BĪŖAgNO3£¬CĪŖCa£ØCH3COO£©2£¬DĪŖAlCl3£¬EĪŖK2CO3£¬FĪŖFeSO4£¬ŅŌ“Ė½ā“šøĆĢā£®
½ā“š ½ā£ŗŅŃÖŖ£ŗŹŅĪĀĻĀ£¬±„ŗĶĒāŃõ»ÆøĘČÜŅŗÅضČŌ¼ĪŖ0.00165 g/mL£¬Ōņ1LČÜŅŗÖŠ×ī¶ąŗ¬ÓŠ1.65gøĘĄė×Ó£¬×ī“óĪļÖŹµÄĮæĪŖ£ŗ$\frac{1.65g}{40g/mol}$=0.04125mol£¬¼“£ŗĒāŃõ»ÆøʵÄ×ī“óÅØ¶ČŠ”ÓŚ0.04125mol/L£¬ŌņĮłÖÖ»ÆŗĻĪļÖŠ²»æÉÄÜŗ¬ÓŠĒāŃõ»ÆøĘ£¬
¢Ł²āµĆČÜŅŗA”¢C”¢E³Ź¼īŠŌ£¬ČżÖÖČÜŅŗĪŖ¼īŅŗ»ņĖ®½ā³Ź¼īŠŌµÄČÜŅŗ£¬ĒŅ¼īŠŌĪŖA£¾E£¾C£¬ŌņAĪŖ¼ī£¬ČÜŅŗÖŠŗ¬ÓŠ“óĮæµÄOH-Ąė×Ó£¬OH-Ąė×ÓÓėAg+£¬Ca2+£¬Fe2+£¬Al3+µČĄė×Ó²»ÄÜ“óĮæ¹²“ę£¬¹ŹAÖ»ÄÜĪŖBa£ØOH£©2£¬øł¾ŻŌ½ČõŌ½Ė®½ā£¬EÓ¦ĪŖĢ¼ĖįŃĪ£¬øł¾ŻĄė×Ó¹²“ę£¬Ö»ÄÜĪŖK2CO3£¬CĪŖ“×ĖįŃĪ£»
¢ŚĻņBČÜŅŗÖŠµĪ¼ÓNa2SČÜŅŗ£¬³öĻÖÄŃČÜÓŚĒæĖįĒŅŅõŃōĄė×ÓøöŹż±ČĪŖ1£ŗ2µÄŗŚÉ«³Įµķ£¬øĆŗŚÉ«³ĮµķĪŖĮņ»ÆŅų£¬ŌņBĪŖĻõĖįŅųČÜŅŗ£»
¢ŪĻņDČÜŅŗÖŠµĪ¼ÓBa£ØNO3£©2ČÜŅŗ£¬ĪŽĆ÷ĻŌĻÖĻó£¬ĖµĆ÷BÖŠ²»ŗ¬SO42-Ąė×Ó£»
¢ÜĻņFČÜŅŗÖŠµĪ¼Ó°±Ė®£¬Éś³É°×É«Šõד³Įµķ£¬³ĮµķŃøĖŁ±ä³É»ŅĀĢÉ«£¬×īŗó±ä³ÉŗģŗÖÉ«£¬ĖµĆ÷FÖŠŗ¬ÓŠFe2+Ąė×Ó£¬
×ŪÉĻ·ÖĪöæÉÖŖ£¬AĪŖBa£ØOH£©2£¬BĪŖAgNO3£¬CĪŖCa£ØCH3COO£©2£¬DĪŖAlCl3£¬EĪŖK2CO3£¬FĪŖFeSO4£¬
£Ø1£©øł¾Ż·ÖĪöæÉÖŖ£¬AĪŖBa£ØOH£©2£¬BĪŖAgNO3£¬CĪŖCa£ØCH3COO£©2£¬
¹Ź“š°øĪŖ£ŗBa£ØOH£©2£»AgNO3£»Ca£ØCH3COO£©2£»
£Ø2£©ĒāŃõ»ÆŃĒĢśŌŚČÜŅŗÖŠ±»ŃõĘųŃõ»Æ³ÉĒāŃõ»ÆĢś£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ4Fe£ØOH£©2+O2+2H2O=4Fe£ØOH£©3£¬
¹Ź“š°øĪŖ£ŗ4Fe£ØOH£©2+O2+2H2O=4Fe£ØOH£©3£»
£Ø3£©DĪŖAlCl3£¬EĪŖK2CO3£¬¶žÕß»ģŗĻŗó·¢ÉśĖ«Ė®½ā·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³ĮµķŗĶ¶žŃõ»ÆĢ¼ĘųĢ壬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ2Al3++3CO32-+3H2O=2Al£ØOH£©3”ż+3CO2”ü£¬
¹Ź“š°øĪŖ£ŗ2Al3++3CO32-+3H2O=2Al£ØOH£©3”ż+3CO2”ü£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄ¼ų±šŗĶ¼ģŃ飬ĪŖøßĘµæ¼µć£¬ĢāÄæÄѶČÖŠµČ£¬°ŃĪÕĪļÖŹÖ®¼äµÄ·“Ó¦”¢ĪļÖŹµÄŠŌÖŹĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪö”¢¼ĘĖć¼°ĶʶĻÄÜĮ¦µÄ漲飬עŅāĄė×Ó¹²“ę¼°µēŗÉŹŲŗćµÄÓ¦ÓĆ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| µēĄėÄÜ/kJ•mol-1 | I1 | I2 |
| Ķ | 746 | 1958 |
| Šæ | 906 | 1733 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗ¬“óĮæFe3+µÄČÜŅŗÖŠ£ŗNH4+”¢Mg2+”¢Cl-”¢HSO3- | |
| B£® | ¼ÓČėAlÓŠH2Éś³ÉµÄČÜŅŗÖŠ£ŗNa+”¢NH4+”¢Cl-”¢NO3- | |
| C£® | NaOHČÜŅŗÖŠ£ŗK+”¢Na+”¢AlO2-”¢CO32- | |
| D£® | NaHCO3ČÜŅŗÖŠ£ŗK+”¢Al3+”¢Cl-”¢SO42- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µē½āŹ±£¬ŹÆÄ«×÷Ņõ¼«£¬²»ŠāøÖ×÷Ńō¼« | |
| B£® | µē½āŹ±£¬Ńō¼«·“Ó¦ŹĒ£ŗI--6e-+3H2OØTIO3-+6H+ | |
| C£® | ČÜŅŗµ÷½ŚÖĮĒæĖįŠŌ£¬¶ŌÉś²ś²»Ąū | |
| D£® | µē½āŗóŅõ¼«ÖÜĪ§ČÜŅŗµÄpHÉżøß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ü | B£® | ¢Ś¢Ū¢Ż | C£® | ¢Ł¢Ś¢Ż | D£® | ¢Ś¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 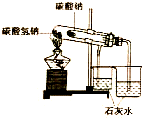 ×°ÖĆæÉÓĆÓŚ±Č½ĻNaHCO3ŗĶNa2CO3µÄĪČ¶ØŠŌ | |
| B£® | 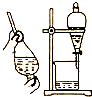 ÓĆ¾Ę¾«ŻĶČ”äåĖ®ÖŠµÄäåæÉŃ”Ōń×°ÖĆ | |
| C£® |  ČēĶ¼×°ÖĆ½ųŠŠŹµŃéæÉ擵½ĖįŠŌKMnO4ČÜŅŗĶŹÉ« | |
| D£® | 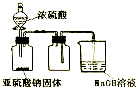 ČēĶ¼×°ÖĆæÉÓĆÓŚŹµŃéŹŅÖĘČ”²¢ŹÕ¼ÆÉŁĮæSO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2.44 kJ | B£® | 44 kJ | C£® | 88 kJ | D£® | 330 kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | s¹ģµĄ³ŹŌ²ŠĪ£¬p¹ģµĄ³ŹŃĘĮåŠĪ | |
| B£® | CuŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķµÄdsĒų | |
| C£® | 1.5g CH3+ÖŠŗ¬ÓŠµÄµē×ÓŹżĪŖ0.8NA | |
| D£® | DNAÖŠµÄ¼ī»ł»„²¹Åä¶ŌŹĒĶعżĒā¼üĄ“ŹµĻÖµÄ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com