
 ��ƻ�����㽶��ˮ���Ĺ����д�����������������ij��ѧ������ȤС�����������������Ϊԭ�Ϻϳ�������������ʵ�鲽�����£�
��ƻ�����㽶��ˮ���Ĺ����д�����������������ij��ѧ������ȤС�����������������Ϊԭ�Ϻϳ�������������ʵ�鲽�����£����� ��1���������������Ũ���������·���������Ӧ����������������
��2������ʱ��Ӧ����ʹ����ˮ���������ܣ��Գ��������
��3��������ӦΪ���淴Ӧ�������������������ƽ�������ƶ�������Ӧ��������Ũ�ȡ����ʵ����Ȳ��ٸı�ʱ�ﵽƽ��״̬��
��4�������������Ʊ�������������������ӷ����������������У�̼������Һ��Ϊ��ϴȥ�����������ᣮ
��� �⣺��1����������������Ӧʱ�����ǻ�������ˮ������������������ˮ���䷴Ӧ����ʽ��CH3COOH+HO��CH2��3CH3 $?_{��}^{Ũ����}$CH3COO��CH2��3CH3+H2O��
�ʴ�Ϊ��CH3COOH+HO��CH2��3CH3 $?_{��}^{Ũ����}$CH3COO��CH2��3CH3+H2O��
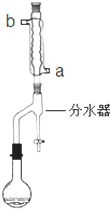
��2����ȴװ��ˮ����Ҫ����������ˮ��a����b����
�ʴ�Ϊ��a��
��3��������ӦΪ���淴Ӧ�������������������ƽ�������ƶ�������Ӧ��������Ũ�ȡ����ʵ����Ȳ��ٸı�ʱ�ﵽƽ��״̬�����ˮ���е�ˮ�㲻������ʱ����Ϊ��Ӧ���յ㣬
�ʴ�Ϊ��ʹ�÷�ˮ�������ˮ��ʹƽ�������ƶ�����߷�Ӧ���ʣ���ˮ���е�ˮ�㲻������ʱ����Ϊ��Ӧ���յ㣻
��4����ˮ���ֳ�������������ᡢ�������ķ�ӦҺһ�����Һ©���У���10mL10%Na2CO3ϴ�ӳ�ȥ���ἰ��������������ϴ����ɺ��л���ӷ�Һ©�����϶˵�������ƿ��
�ʴ�Ϊ����ȥ���ἰ���������������ϣ�
���� ���⿼���������Ʊ���ʵ����̷�����ʵ�������ƣ�Ϊ�߿��������ͣ�������ѧ���ķ�����ʵ�������Ŀ��飬���ʷ�����Լ�ѡ����������⣬ʵ�黯ѧ��Դ�ڳ���ʵ��ͻ����������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ba2+��NO3-��NH4+��Cl- | B�� | K+��Ba2+��Cl-��SO42- | ||
| C�� | Al3+��CO32-��NH4+��AlO2- | D�� | Cu2+��NH4+��SO42-��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| �� | �� | �� | �� | |
| ����Һ�������mL�� | 30 | 30 | 30 | 30 |
| ��Ʒ��g�� | 3.32 | 4.15 | 5.81 | 7.47 |
| ������̼�������mL�� | 672 | 840 | 896 | 672 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
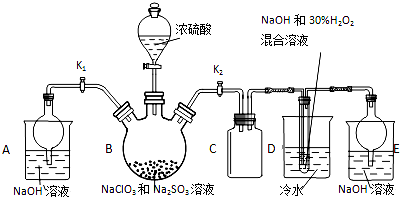 �������ƣ�NaClO2������ҪƯ����̽��С�鿪չ����ʵ�飬��ش�
�������ƣ�NaClO2������ҪƯ����̽��С�鿪չ����ʵ�飬��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
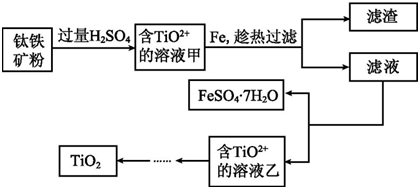
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
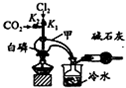 ���Ȼ��ף�PCl3���Ǻϳ�ҩ�����Ҫ����ԭ�ϣ���ͨ�������������ϵõ���
���Ȼ��ף�PCl3���Ǻϳ�ҩ�����Ҫ����ԭ�ϣ���ͨ�������������ϵõ���| ���� | �۵�/�� | �е�/�� | �ܶ�/g•cm-3 |
| ���� | 44.1 | 280.5 | 1.82 |
| PCl3 | -112 | 75.5 | 1.574 |
| POCl3 | 2 | 105.3 | 1.675 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com