
��14�֣�������Ԫ���γɵij����ǽ������嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ����D����ɫ���г�������ζ������E��D��������ǿ�ᣬҲ������ǿ�E���Ӻ�18�����ӣ�������������ȼ������G��G�ڴ������ܵ���������γɡ�E����������������Һ���յõ���ɫ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��������ƵĽṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��1����ɵ���A��Ԫ��λ�����ڱ��е� ���ڵ� �壻
��2��������H�ĵ���ʽΪ ��
��3��B������������Һ��Ӧ�����ӷ���ʽΪ�� ��
��4��G����������Һ��Ӧ����������ɱ�����������ȡ�д����Ӧ�����ӷ���ʽ��
��5����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ�� ��
��6��д��F��Һ�и�����Ũ���ɴ�С�Ĺ�ϵΪ�� ��
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
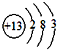
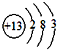
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡΫ��������һУ������ѧ��ģ���⻯ѧ�Ծ� ���ͣ������
(10��)������Ԫ���γɵij����ǽ������嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ��D������E��D��������ǿ�ᣬҲ������ǿ�E������������ȼ�ղ����̼�������G��G�ڴ������ܵ���������γɡ�E����������������Һ���յõ���ɫ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��������ƵĽṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��ش���������:
(1)��ɵ���A��Ԫ�������ڱ��е�λ���� ��
(2)��ɵ���B��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ ��
(3)G�������������������·�Ӧ��������ùɱ������������.�÷�Ӧ��������
Ϊ ��
(4)��ҺF�ڿ����г��ڷ�������H�Ļ�ѧ��Ӧ���ʽΪ: 
��
(5��H����Һ��ϡ���ᷴӦ����������Ϊ 
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ȫ��ͳһ�������ۻ�ѧ���֣��Ĵ����� ���ͣ������
��15�֣�
������Ԫ���γɵij����ǽ������嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ����D������E��D��������ǿ�ᣬҲ������ǿ�E������������ȼ�ղ����̼�������G��G�ڴ������ܵ���������γɡ�E����������������Һ���յõ���ɫ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��������ƵĽṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��ش��������⣺
��1����ɵ���A��Ԫ��λ�����ڱ��е� ���ڣ��� �塣
��2��B������������Һ��Ӧ�Ļ�ѧ����ʽΪ��
��
��1��G�������������������·�Ӧ����������ɱ�����������ȡ��÷�Ӧ����������Ϊ
��������2 ��������ʱ��ת�Ƶ���
��������ʱ��ת�Ƶ���  ��
��
��2����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ��
��
��3��H����Һ��ϡ���ᷴӦ����������Ϊ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com