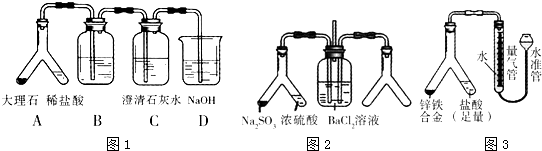
�⣺��1����̽��CO
2�����ʯ��ˮ�ķ�Ӧ��������̼�����к����Ȼ������壬����Ŷ�����̼�ļ��飬��Ҫͨ������̼��������Һ��ȥ�Ȼ��⣬
�ʴ�Ϊ������̼��������Һ��
�ڶ�����̼���岻����Ⱦ���壬���Բ�������װ�ã��ʴ�Ϊ���ǣ�������̼��һ�������壬����Ҫ���գ�
��2��SO
2��BaCl
2��Ӧ������������Һ�б�����ڴ�����SO
32-���Ҳ�Y����Ӧ�����ɼ�����������������壬���Ǽ������壬��Һ�д��ڴ�����SO
32-���������������壬��Һ�п�����SO
42-�������ɵij�������ΪBaSO
3��BaSO
4��
�ʴ�Ϊ��Ũ��ˮ����ʯ�ң����� NaOH����ʯ�ң���BaSO
3��BaSO
4��
��3���ٶ�ȡ������������ʱ��Ϊ��С��Ӧʹˮ�ܡ���������Һ����ƽ���ʴ�Ϊ��̧�ߣ����ƶ���ˮ��λ�ã�ʹˮ�ܡ���������Һ����ƽ��
����Ͻ���FeΪxmol��ZnΪymol������
56x+65y=0.117
x+y=
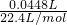
=0.002mol
��֮�ã�x=0.001444��y=0.000556��
��Ͻ������ĺ���Ϊ
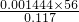
=69.14%��
�ʴ�Ϊ��69.14%��
��������1��̽��CO
2�����ʯ��ˮ�ķ�Ӧ����Ҫ�ų���������ĸ��ţ�������̼�����к����Ȼ�����Ŷ�����̼�ļ��飬������̼������Ⱦ���壬����Ҫβ�����գ�
��2��SO
2��BaCl
2��Ӧ������������Һ�б�����ڴ�����SO
32-��
��3���ٶ�ȡ������������ʱ��Ϊ��С��Ӧʹˮ�ܡ���������Һ����ƽ��
�ڼ���Ͻ���FeΪxmol��ZnΪymol���з���ʽ��ɽ����⣮
���������⿼�����������ʵķ����жϣ�ʵ����֤�������ʵ�ʵ����ƺ�����װ�÷�������ѧ����ʽ���㣬��Ŀ�Ѷ��еȣ�

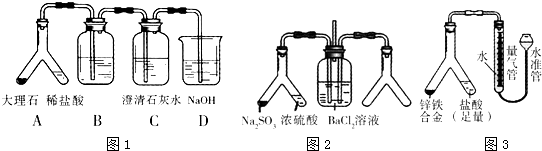
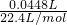 =0.002mol
=0.002mol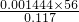 =69.14%��
=69.14%��