
��12�֣�Ϊ�˲ⶨm g�������ƺ�̼���ƵĹ���������̼���Ƶ������������ס���
![]() ��λͬѧ�ֱ���������µ�ʵ�鷽����
��λͬѧ�ֱ���������µ�ʵ�鷽����
��1����ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬��������Ȼ�����Һ�����ˡ�ϴ�ӣ�ȡ������ɣ������ù���ng����������̼���Ƶ���������Ϊ ��
��2����ͬѧ�ķ����ǣ�����Ʒ�ܽ�����Թ������Ȼ�����Һ���ٵ���2�D3�η�̪��Һ���ñ�����ζ�����ͬѧ�ڵζ�����������Ҫ����Ҫ���������� �� ����������Ȼ�����Һ��Ŀ���� ��
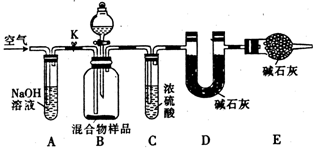
��3����ͬѧ�ķ�������ͼ��ʾ���������Ʒ��ȫ��Ӧʱ������ͨ�������Ŀ���� �����У�װ��A�������� ��װ��E������ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 0.14CV |
| m |
| 14CV |
| m |
| 0.14CV |
| m |
| 14CV |
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
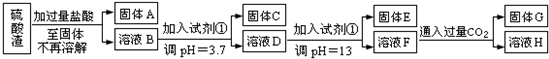
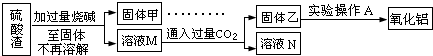
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.��ͬѧ�ķ�������ͼ��ʾ��
(1)���ݼ�ͬѧ��ʵ��װ��ͼ��������ÿ��ʵ������������еij�����������Ҫ����_________�Ρ�
(2)��ͬѧ�ظ��ⶨ�����Σ��õ�̼���Ƶ��������������ݴ��ڽϴ��ƫ�����Ϊԭ�������_________(�����)��
A.װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B.װ��������е�ˮ�����Ͷ�����̼����ʯ������
C.��Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ������
D.����ϡ����������㣬�����������
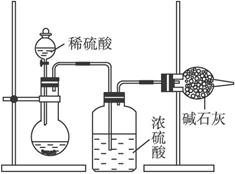
��.��ͬѧ�ķ����ǣ���ȡ��Ʒm g�����ܽ⣬�ӹ����Ȼ�����Һ�����ˡ�ϴ�ӡ���ɣ������ù���n g��
(1)�������̼���Ƶ���������Ϊ(��m��n��ʾ) ___________________________��
(2)ϴ�ӳ����IJ���Ҫ����___________________________��
(3)Ca2+��Ba2+������ʹ![]() ������ȫ������ͬѧʹ���Ȼ�����Һ�������Ȼ�����Һ��ԭ����___________________________���ⶨ
������ȫ������ͬѧʹ���Ȼ�����Һ�������Ȼ�����Һ��ԭ����___________________________���ⶨ![]() ��������ʹ������������Һ����������������Һ����������и��ߵľ�ȷ�ȣ�ԭ����___________________________��
��������ʹ������������Һ����������������Һ����������и��ߵľ�ȷ�ȣ�ԭ����___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ͳ���ģ ���ͣ��ʴ���


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com