


 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
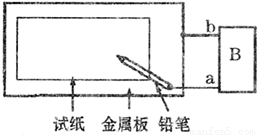
 ��ͼ��ʾ��ȡһ���ñ��͵�NaCl��Һ��ʪ��pH��ֽ������Ǧ��о���缫����ֱͨ����Դ��һ��ʱ�����a�缫����ֽ�Ӵ�������һ��˫ɫͬ��Բ����ȦΪ��ɫ����Ȧ��dz��ɫ��������˵��������ǣ�������
��ͼ��ʾ��ȡһ���ñ��͵�NaCl��Һ��ʪ��pH��ֽ������Ǧ��о���缫����ֱͨ����Դ��һ��ʱ�����a�缫����ֽ�Ӵ�������һ��˫ɫͬ��Բ����ȦΪ��ɫ����Ȧ��dz��ɫ��������˵��������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
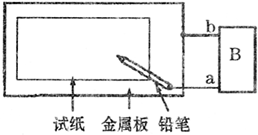 ȡһ���ñ��͵�NaCl��Һ��ʪ��ʯ����ֽ��ƽ����һ��������ϣ�����ͼ��ʾ�ķ������ӵ�·��
ȡһ���ñ��͵�NaCl��Һ��ʪ��ʯ����ֽ��ƽ����һ��������ϣ�����ͼ��ʾ�ķ������ӵ�·���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��08���Ϻ�����ȡһ���ñ��͵�NaCl��Һ��ʪ��pH��ֽ������Ǧ��о���缫����ֱͨ����Դ��һ��ʱ�����a�缫����ֽ�Ӵ�������һ��˫ɫͬ��Բ����ȦΪ��ɫ����Ȧ��dz��ɫ��������˵��������� �� ��
��08���Ϻ�����ȡһ���ñ��͵�NaCl��Һ��ʪ��pH��ֽ������Ǧ��о���缫����ֱͨ����Դ��һ��ʱ�����a�缫����ֽ�Ӵ�������һ��˫ɫͬ��Բ����ȦΪ��ɫ����Ȧ��dz��ɫ��������˵��������� �� ��
A��b�缫������ B��a�缫���Դ����������
C����������ˮ�������� D��b�缫������Һ��pH��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡһ���ñ��͵�NaCl��Һ��ʪ��pH��ֽ������Ǧ��о���缫����ֱͨ����Դ��һ��ʱ�����a�缫����ֽ�Ӵ�������һ��˫ɫͬ��Բ����ȦΪ��ɫ����Ȧ��dz��ɫ��������˵���������( )

A��b�缫������ B��a�缫���Դ����������
C����������ˮ�������� D��b�缫������Һ��pH��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ѡ����
ȡһ���ñ��͵�NaCl��Һ��ʪ��pH��ֽ������Ǧ��о���缫����ֱͨ����Դ��һ��ʱ�����a�缫����ֽ�Ӵ�������һ��˫ɫͬ��Բ����ȦΪ��ɫ����Ȧ��dz��ɫ��������˵���������( )
A��b�缫������ B��a�缫���Դ����������
C����������ˮ�������� D��b�缫������Һ��pH��С
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com