
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��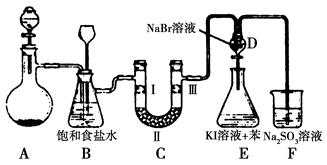
(1)�Ʊ�����ѡ�õ�ҩƷΪƯ�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ________��
(2)װ��B�б���ʳ��ˮ��������________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����__________________��
(3)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η������ʵ������________(����)��
| ��� | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
S2C12��һ�ֽ��ɫ�ӷ���Һ�壬������������ij��ѧ��ȤС�������ʵ���Ʊ�������S2C12����������֪S2Cl2��ˮ�������绯��Ӧ��һ������Ԫ�ػ��ϼ����ߣ���һ���ֻ��ϼ۽��ͣ����������������ʺ��������Cl2��Ӧ��������S2C12����Ӧ�Ļ�ѧ����ʽΪ��2S+Cl2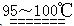 S2Cl2��
S2Cl2��
��Ӧ�漰�ļ������ʵ��۷е����£�
| ���� | S | S2Cl2 |
| �е�/�� | 445 | 138 |
| �۵�/�� | 113 | -76 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ij�о���ѧϰС�������ȡ������������Ϊ��Ӧ������ض���Ӧ��װ�á�
(1)Ҫ��Cװ�ý���B��D֮�䣬��ȷ�Ľӷ��ǣ�a�� �� ��d��
(2)ʵ�鿪ʼ�ȵ�ȼA���ľƾ��ƣ���K����Cl2��������װ�ã��ٵ�ȼD���ľƾ��ơ�Cl2ͨ��Cװ�ú����D��Dװ����ʢ��̼�ۣ�����������ԭ��Ӧ������CO2��HCl(g)��������Ӧ�Ļ�ѧ����ʽΪ ��
(3)D����Ӧ��Ϻرշ�K����ȥ�����ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ��
(4)��A��B��C��D��Eװ������һ����Ҫ�Ľ���˵����Ҫ�Ľ������ɲ������Ľ����װ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij������ȤС��Ϊ̽��ͭ��Ũ���ᷴӦ���������ͼ��ʾװ�ý���ʵ�顣��֪����SO2�����ڱ���������������Һ����SO2�������Ը��������Һ����������ԭ��Ӧʹ֮��ɫ����ѧ����ʽΪ5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4����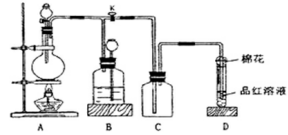
�ش��������⣨ע��EΪֹˮ�У�FΪ��������
��1�����Aװ�õ������Եķ��� ��
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3��װ��D���Թܿڷ��õ���Ӧպ��NaOH��Һ��
�������� ��
��4��װ��B����������������á���D�������Ե�����ر�����F����ȥ�ƾ��ƣ��������ȵ����ã�A�����������������ʱB�е������� ��B��Ӧ���õ�Һ���ǣ�����ĸ�� ��
| A��ˮ | B������NaHSO3��Һ | C������KMnO4��Һ | D��NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���Ҳⶨ�����Һ��I���ĺ����Լ���Ļ��չ������£�
��.�����Һ��I�������IJⶨ
����Һ����ȡ25.00 mL��Һ��250 mL��ƿ�У��ֱ����5 mL 2 mol��L��1 H2SO4��10 mL 20% NH4Fe(SO4)2��12H2O��Һ��ҡ�ȡ�С���������������ȫ������ȡ����ƿ��ȴ����10 mL 2 mol��L��1H2SO4�����뼸�ζ�����������(����ָʾ��)����0.0250 mol��L��1��K2Cr2O7��Һ���еζ����յ㡣�ظ�3�Σ����ݼ�¼���±���
(��֪��Ӧ����2Fe3����2I��=2Fe2����I2����6Fe2����Cr2O72-��14H��=6Fe3����2Cr3����7H2O)
| ���� | 1 | 2 | 3 |
| ����(mL) | 19.60 | 19.65 | 19.55 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
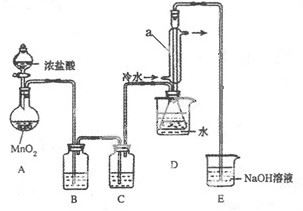
����������������������ע����Һ���ش��������⡣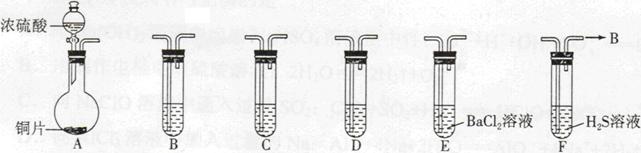
��1������װ��A��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ����ƿ�е�ʵ������Ϊ ��
��2����֤̼����ǽ����Ե����ǿ��(��֪���ԣ�H2SO3>H2CO3)����ѡ������A��B��C��D�������ӣ���B��C��D����ѡ����Լ��ֱ�Ϊ �� �� ����˵��̼�ķǽ����Աȹ�ǿ��ʵ�������� ��
��3����֤SO2�������ԡ���ԭ�ԡ���ѡ��A��E��F����������A��E��F˳�����ӡ�
����֤��SO2�������Ե�ʵ�������� ����Ӧ����ʽΪ��
��
����֤��SO2���л�ԭ�ԣ���E�в�ȡ��ʵ�����Ϊ ����ʵ������Ϊ ����Ӧԭ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
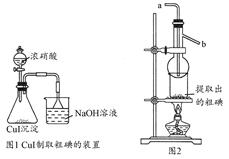
ij����С��������CuO��NH3��Ӧ,�о�NH3��ij�����ʲ��ⶨ�����,���������ʵ��װ��(�г�װ��δ����)����ʵ�顣��ش���������: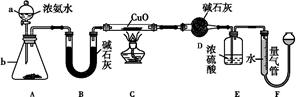
(1)����a������Ϊ��������;����b�п�ѡ����Լ�Ϊ����������
(2)ʵ������,����װ��A,������ȡ����ɫ�������������� (����ĸ)��
| A��Cl2 | B��O2 | C��CO2 | D��NO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ��ʵ���ҳ�����ʱ���Ƶ�һ���Լ�,ͼ����ʾ����ʵ����������ˮʱ��һ�ֳ���װ��,ͼ����ij��ѧʵ��С��������Ƶ�һ��������ˮ��װ��(ͼ�еĹ�������һ�־������嵥�ġ���������ƿ�й������������)��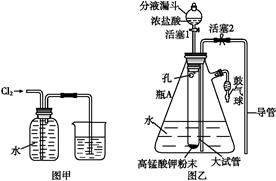
(1)��ˮ����ʱ���Ƶ�ԭ����: ��(�û�ѧ����ʽ��ʾ)��
(2)ͼ����ʾװ����,�ձ��ڵ�Һ��������������
(3)ͼ����ʾװ����,ƿA �ڵĴ��Թ��϶˹ܱ�����һ��С��,ƿA�����ƽ���������,������ͨ������
��������ʵ����(�����ʵ�����������)��
(4)��ͼ����ʾװ��������ˮʱ,������������:
�ٹرջ���2,��Һ©���ϿڵIJ�����,�ٴ���1,������Ũ����ע����Թ��ڡ�
�ڹرջ���1,���Ϸ�Һ©���Ͽڲ�������Ũ����ʹ��Թ��ڵĸ�����ط�ĩ��Ӧ����������
������ҡ��ƿA,ʹ������������ˮ�С�
��ͼ��װ�����ڽ϶�ʱ���ڵõ�������ˮ��������ҡ�������������������ˮ�ĽӴ�������,��һ����Ҫԭ���� ��
��Һ©���е�����Ӧ�����μ����Թ��ڡ���һ�μ���̫�������,��������ĺ���� ��
(5)������ˮ��ɺ�,���ز�жװ�ü��ɴ�ƿA��ȡ��������ˮ,������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ5��װ�ö�����ѧ��ѧ�г�����ʵ��װ�ã�ijѧϰС���ͬѧ������Щװ�ý��г������ʵ���ȡ��̽�������ʣ�ͼ��a��b��c��ʾֹˮ�У��������������ƻ����ۣ��Իش��������⣺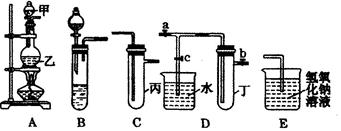
��1����A��C��E��������Ũ����� ����д���ƣ�Ϊԭ����ȡCl2�������ҵ������� ��
��2�����ã�1����װ�ú�ҩƷ���ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ��������ˮ����Ʒ����Һ | Ʒ����Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��������ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ���ٲ���һ������ǿ��̼������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com