
(12��)��A��B��C��D���ֶ����ڵķǽ���Ԫ��(�䵥��Ҳ�ɷֱ���A��B��C��D��ʾ)������Ԫ�ص�ԭ��������B��D��C��A˳������D��CԪ�������ڱ���λ�����ڡ���һ�������£�B���Էֱ��A��C��D�������ɼס��ҡ��������C��D���Ͽɵö�����֪�ҡ������������и�����10�����ӣ����Ҽס��ҡ����������������µı仯��ϵ��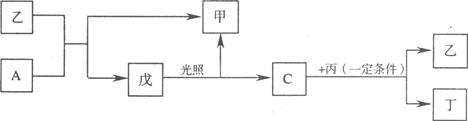
����д���пո�
(1)��Ũ��Һ��һ�ֺ�ɫ��ĩ���ȿɵ�A�����Ϊ_______����Ϊ___________��
(2)��Ľṹʽ��______________��DB4+�ĵ���ʽ��_______________��
(3)��+����D��һ�������·�Ӧ����һ��������Ⱦ�ĺð취��д���÷�Ӧ�Ļ�ѧ����ʽ��
����������ת�������_____________________________________________________
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)��A��B��C��D����Ԫ�أ�AԪ���γɵ�-2�������ӱȺ�ԭ�ӵĺ����������8����B Ԫ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʣ�CΪԭ�Ӻ�����12�����ӵĶ��۽�������2 .4��C��������ˮ��Ӧʱ���ڱ�״���·ų�����2.24L��D��M����7�����ӡ����٢ڢ�ÿ��1�֣�
��д��A~DԪ�ط��ţ�A____,B_____,C_____,D_____
��д��A��ԭ�ӽṹʾ��ͼ�� B�����ӽṹʾ��ͼ:��
D�����ڱ���λ�ã� C��D���γɵĻ�����ĵ���ʽ
���ֱ�д��B ��D������������ˮ����Ļ�ѧʽ______ ��______
���Ƚ�D����̬�⻯����H2S��HF���ȶ��ԣ�(2��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����������һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��ÿ��2�֣���12�֣�
����A��B��C��D��E��F�������ʻ����ӣ�����A��B��C��D��������ͼ��ʾ�Ľṹ��ṹ��Ԫ����ͼ������������������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����A��B�ľ���������ͬ������A��ͬ������������B���ʷ����û���Ӧ��C��D��E��F������ȵĵ���������D�������ӣ�D��F�����Ԫ����ͬ��C��E��F�ľ���������ͬ����E���ɵ����ʳ����³�Һ̬����ش��������⣺
��1��д������A��ͬ����������B���ʷ����û���Ӧ�Ļ�ѧ����ʽ______ ��ͬ����ĵ�������Ԫ�ػ�̬ԭ�ӵ���Χ�����Ų�ʽΪ ��
��2�������������ʻ����ӵ����Ԫ���������ִ���ͬһ���ڣ���д��������Ԫ�ص�һ�������ɴ�С��˳�� ��
��3�������������ʻ������л�Ϊ�ȵ�������� ��д����ѧʽ����
��4��F���ӵ�����ԭ���ӻ������� ��F������E����ԭ����
________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ĸ߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��ÿ��2�֣���12�֣�
����A��B��C��D��E��F�������ʻ����ӣ�����A��B��C��D��������ͼ��ʾ�Ľṹ��ṹ��Ԫ����ͼ������������������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����A��B�ľ���������ͬ������A��ͬ������������B���ʷ����û���Ӧ��C��D��E��F������ȵĵ���������D�������ӣ�D��F�����Ԫ����ͬ��C��E��F�ľ���������ͬ����E���ɵ����ʳ����³�Һ̬����ش��������⣺

��1��д������A��ͬ����������B���ʷ����û���Ӧ�Ļ�ѧ����ʽ______ ��ͬ����ĵ�������Ԫ�ػ�̬ԭ�ӵ���Χ�����Ų�ʽΪ ��
��2�������������ʻ����ӵ����Ԫ���������ִ���ͬһ���ڣ���д��������Ԫ�ص�һ�������ɴ�С��˳�� ��
��3�������������ʻ������л�Ϊ�ȵ�������� ��д����ѧʽ����
��4��F���ӵ�����ԭ���ӻ������� ��F������E����ԭ����
________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ����
��ѡ��ģ������ݡ���ѡ������һ�����������ⶼ������A�����֡�
A.��A��B��CΪ���ӣ�D��FΪ�����ӣ�EΪ�����ӣ����Ƕ�����lO�����ӣ�B��
��A�����õ����ʿɵ����D��E��C����Ҫ�Ļ�ʯ��Դ����A��B�ͺ�F���ӵ���
�ʻ�Ϻ�ɵ�D��һ�ְ�ɫ������![]() ������Arԭ�ӵĵ��Ӳ�ṹ��ͬ����ش�:
������Arԭ�ӵĵ��Ӳ�ṹ��ͬ����ش�:
(1)��̬Gԭ�ӵ���Χ�����Ų�ʽ�� ����A��B��C�����ַ����У����ڷǼ�
�Է��ӵ��� (д��ѧʽ)��
(2)�����й�B��˵������ȷ���� ��(����ĸ)
a����������п���Ϊ���� b���÷��ӵ��ȶ���������й�
c�������ɸ�ԭ��������Ϊ8![]() �����ȶ��ṹ
�����ȶ��ṹ
d����1 mol B��Һ������3 mol���
(3)���ݵȵ�����ԭ����D���ӵĿռ乹���� ��������ԭ�ӹ�����ӻ�����
 �� ��
�� ��
(4)����C������ԭ�ӿ����γɶ��ֵ��ʣ�������һ��Ϊ�ռ���״��
������ͼ���������ĵġ���ʾ�þ����е�һ��ԭ�ӣ����ڸ�
������Ķ������á���ʾ����֮���ڵ�ԭ�ӡ�
(5)����֤ʵF���ռ���Һ��Ӧ��Na[F(OH)4]���ɣ���Na[F(OH)4]��
������ ��(����ĸ)
a�������� b�����Ӽ� c���Ǽ��Լ� d�����Լ�
f.��� g��![]() �� h��
�� h��![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�����������ⶼ��,��A�����֡�
A��CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ���֪��
��CuCl������CuCl2���ʵ��Ļ�ԭ����SO2��SnCl2�Ȼ�ԭ�Ƶã�
![]()
��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�
��ش��������⣺
(1)��̬Cuԭ�ӵĺ�������Ų�ʽΪ_________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����_____��
(2)SO2���ӵĿռ乹��Ϊ____________����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ_________��
 (3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_____________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_______________________________________��
(3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_____________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_______________________________________��
(4)�������γɵ��������к��еĻ�ѧ��������__________��(����ĸ)
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
(5)CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com