
���� ��1��������Һ��ϡ���������ʵ����ʵ�������������ҪŨ����������
��2����������100mL 0.9mol/L��ϡ����IJ���Ը�������������
��3����������ƿ��ʹ�÷�����ԭ�����ش�
��4������c=$\frac{n}{V}$�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��� �⣺��1����9mol/L��Ũ����ϡ�ͳ�0.9mol/L��ϡ����100mL������n=c1V1=c2V2��֪����ҪŨ��������Ϊ��$\frac{0.9mol/L��0.1L}{9mol/L}$=0.01L=10mL���ʴ�Ϊ��10��
��2������100mL 0.9mol/L��ϡ����IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݺ�ҡ�ȵȣ���ȷ�IJ���˳��Ϊ��CDBAGFE��
�ʴ�Ϊ��CDBAGFE��
��3������ƿ��ʹ�÷�������ʹ��֮ǰ�����ȼ����Ƿ�©ˮ���ʴ�Ϊ������Ƿ�©ˮ��
��4��A��ϡ�ͺ�û����ȴ����ж��ݣ��൱�ڼ���ˮ����ƫ�٣�����Ũ��ƫ�ߣ���A����
B��û��������ˮϴ���ձ��Ͳ��������൱�����ʱ���ģ�n��С��Ũ�Ƚ��ƫ�ͣ���B��ȷ��
C������ƿ������ˮϴû�к�ɣ�����Ӱ����Һ����������ʵ����ʵ�������������ܵ�Ӱ�죬��C����
D������õ���Һ����������ˮϴ����δ�ɵ��Լ�ƿ�б��ã��൱�ڽ���õ���Һϡ�ͣ�Ũ��ƫ�ͣ���D��ȷ��
E������ʱ�μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ�����������ˮʹҺ�氼����̶������У�����ˮ����ƫ�࣬�൱�ڽ���õ���Һϡ�ͣ�Ũ��ƫ�ͣ���E��ȷ��
��ѡBDE��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ������Ѷ��еȣ������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������ע����ȷ�������ķ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 21% | B�� | 32% | C�� | 37% | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2Br | B�� | CH2�TCHBr | C�� | CH3CH2OH | D�� | 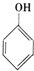 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g��+49.0kJ��ij��ѧʵ�齫6mol CO2��8mol H2����һ�ݻ�Ϊ2L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ��ʵ����ʾ��ͼ����ĸ������ֱ�ʾ��Ӧ�����꣩���ش��������⣺
�̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g��+49.0kJ��ij��ѧʵ�齫6mol CO2��8mol H2����һ�ݻ�Ϊ2L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ��ʵ����ʾ��ͼ����ĸ������ֱ�ʾ��Ӧ�����꣩���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O��Na2O2������ˮ�������Ǽ��������� | |
| B�� | Na2O��Na2O2������ˮ�������Ϸ�Ӧ������O2 | |
| C�� | Na��Na2O��Na2O2����ˮ���γɵ���Һ��������ͬ | |
| D�� | Na�Ż������ĭ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ���ż� | B�� |  ������ | C�� |  ����ǯ | D�� |  ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
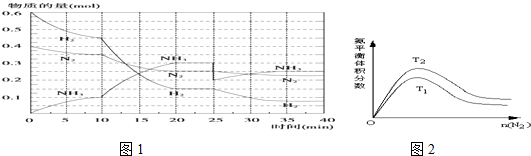
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4L��NO2��NO��ɵĻ�������к��е���ԭ����ĿΪ2NA | |
| B�� | ���³�ѹ�£���34gH2O2����Һ����ԭ�ӵ����ʵ���Ϊ2moI | |
| C�� | �����£�16.8gFe������ˮ������ȫ��Ӧ��ʧȥ0.8NA������ | |
| D�� | �����£�100mLlmol��L-l������4.6 g�Ʒ�Ӧ����0.05 molH2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com