
خھءث·ہضخ»·¾³خغب¾²¢¶شخ²ئّ½ّذذ×غ؛دہûسأ£¬ؤ³ءٍثل³§سأ°±ث®خüتصخ²ئّضذµؤSO2£¬شظدٍخüتصز؛ضذ¼سبëإ¨ءٍثل£¬زشضئب،¸كإ¨¶بµؤSO2¼°£¨NH4£©2SO4؛حNH4HSO4¹ججه،£
خھءث²â¶¨ةدتِ£¨NH4£©2SO4؛حNH4HSO4¹ججه»ى؛دخïµؤ×é³ة£¬دض³ئب،¸أرùئ·ثؤ·ف£¬·ض±ً¼سبëدàح¬إ¨¶بµؤNaOHبـز؛¸÷40.00mL£¬¼سببضء120،و×َسز£¬ت¹°±ئّب«²؟زف³ِ[(NH4)2SO4؛حNH4HSO4µؤ·ض½âخآ¶ب¾ù¸كسع200،و]£¬²âµأسذ¹طتµرéت¾فبçدآ£¨±ê×¼×´؟ِ£©£؛
£¨1£©تµرé¹³جضذسذ¹ط·´س¦µؤہë×س·½³جت½خھ
£»
£¨2£©سةI×éت¾فض±½سحئ²â£؛±ê×¼×´؟ِدآ3.7gرùئ·½ّذذح¬رùتµرéت±£¬ةْ³ة°±ئّµؤجه»خھ
L£»
£¨3£©تش¼ئثم¸أ»ى؛دخïضذ(NH4)2SO4؛حNH4HSO4µؤخïضتµؤء؟ض®±ب £»
£¨4£©سû¼ئثم¸أNaOHبـز؛µؤخïضتµؤء؟إ¨¶بس¦ر،شٌµع ×éت¾ف£¬سة´ثاَµأNaOHبـز؛µؤخïضتµؤء؟إ¨¶بخھ ،£

| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
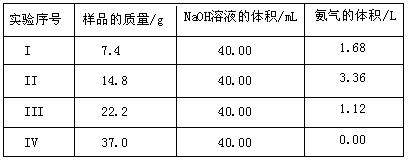
تµرéذٍ؛إ | رùئ·µؤضتء؟/g | NaOHبـز؛µؤجه»/mL | °±ئّµؤجه»/L |
¢ٌ | 7.4 | 40.00 | 1.68 |
¢ٍ | 14.8 | 40.00 | 3.36 |
¢َ | 22.2 | 40.00 | 1.12 |
¢ô | 37.0 | 40.00 | 0.00 |
£¨1£©ذ´³ِتµرé¹³جضذسذ¹طµؤہë×س·½³جت½،£
£¨2£©سة±ي¸ٌت¾فض±½سحئ²â±ê×¼×´؟ِدآ3.7 gرùئ·½ّذذح¬رùتµرéت±£¬ةْ³ة°±ئّµؤجه»خھ¶àةظة£؟
£¨3£©تش¼ئثم¸أ»ى؛دخïضذ(NH4)2SO4؛حNH4HSO4µؤخïضتµؤء؟ض®±ب،£
£¨4£©سû¼ئثم¸أNaOHبـز؛µؤخïضتµؤء؟إ¨£¬¶بس¦ر،شٌµع_________×éت¾ف£¬سة´ثاَµأNaOHبـز؛µؤخïضتµؤء؟إ¨¶بخھ¶àةظ£؟
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
خھءث·ہضخ»·¾³خغب¾²¢¶شخ²ئّ½ّذذ×غ؛دہûسأ£¬ؤ³ءٍثل³§سأ°±ث®خüتصخ²ئّضذµؤSO2£¬شظدٍخüتصز؛ضذ¼سبëإ¨ءٍثل£¬زشضئب،¸كإ¨¶بµؤSO2¼°(NH4)2SO4؛حNH4HSO4¹ججه،£خھ²â¶¨ةدتِ(NH4)2SO4؛حNH4HSO4¹ججه»ى؛دخïµؤ×é³ة£¬دض³ئب،رùئ·ثؤ·ف£¬·ض±ً¼سبëدàح¬إ¨¶بµؤNaOHبـز؛¸÷40.00 mL£¬¼سببضء120 ،و×َسز£¬ت¹°±ئّب«²؟زف³ِ،²(NH4)2SO4؛حNH4HSO4·ض½âخآ¶ب¾ù¸كسع200 ،و،³£¬²âµأسذ¹طتµرéت¾فبçدآ£¨±ê×¼×´؟ِ£©£؛
| تµرéذٍ؛إ | رùئ·µؤضتء؟/g | NaOHبـز؛µؤجه»/mL | °±ئّµؤجه»/L |
| ¢ٌ | 7.4 | 40.00 | 1.68 |
| ¢ٍ | 14.8 | 40.00 | 3.36 |
| ¢َ | 22.2 | 40.00 | 1.12 |
| ¢ô | 37.0 | 40.00 | 0.00 |
£¨1£©ذ´³ِتµرé¹³جضذسذ¹طµؤہë×س·½³جت½،£
£¨2£©سة±ي¸ٌت¾فض±½سحئ²â±ê×¼×´؟ِدآ3.7 gرùئ·½ّذذح¬رùتµرéت±£¬ةْ³ة°±ئّµؤجه»خھ¶àةظة£؟
£¨3£©تش¼ئثم¸أ»ى؛دخïضذ(NH4)2SO4؛حNH4HSO4µؤخïضتµؤء؟ض®±ب،£
£¨4£©سû¼ئثم¸أNaOHبـز؛µؤخïضتµؤء؟إ¨£¬¶بس¦ر،شٌµع_________×éت¾ف£¬سة´ثاَµأNaOHبـز؛µؤخïضتµؤء؟إ¨¶بخھ¶àةظ£؟
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
خھءث·ہضخ»·¾³خغب¾²¢¶شخ²ئّ½ّذذ×غ؛دہûسأ£¬ؤ³ءٍثل³§سأ°±ث®خüتصخ²ئّضذµؤSO2£¬شظدٍخüتصز؛ضذ¼سبëإ¨ءٍثل£¬زش»طتص¸كإ¨¶بµؤSO2²¢µأµ½¸±²ْئ·»¯·ت(NH4)2SO4؛حNH4HSO4،£خھ²â¶¨ةدتِ(NH4)2SO4؛حNH4HSO4¹ججه»ى؛دخïµؤ×é³ة£¬دض³ئب،¸أرùئ·ثؤ·ف£¬·ض±ً¼سبëدàح¬إ¨¶بµؤNaOHبـز؛¸÷40.00mL£¬¼سببضء120،و×َسز£¬ت¹°±ئّب«²؟زف³ِ£غزرضھ£؛(NH4)2SO4؛حNH4HSO4µؤ·ض½âخآ¶ب¾ù¸كسع200،و£ف£¬²âµأسذ¹طتµرéت¾فبçدآ£¨±ê×¼×´؟ِ£©£؛
| تµرéذٍ؛إ | رùئ·µؤضتء؟/g | NaOHبـز؛µؤجه»/mL | °±ئّµؤ |
| ¢ٌ | 7.4 | 40.00 | 1.68 |
| ¢ٍ | 14.8 | 40.00 | 3.36 |
| ¢َ | 22.2 | 40.00 | 1.12 |
| ¢ô | 37.0 | 40.00 | 0 |
£¨1£©²â¶¨¹³جضذسذ¹ط·´س¦µؤہë×س·½³جت½خھ
_____________________________ ،£
£¨2£©3.7g¸أرùئ·½ّذذح¬رùتµرéت±£¬ةْ³ةµؤ°±ئّشع±ê×¼×´؟ِدآجه»خھ__________L،£
£¨3£©تشاَثم¸أ»ى؛دخïضذ(NH4)2SO4؛حNH4HSO4µؤخïضتµؤء؟ض®±ب،£
£¨4£©سû¼ئثم¸أNaOHبـز؛µؤخïضتµؤء؟إ¨¶بس¦ر،شٌµع______×éت¾ف£¬²¢سة´ث¼ئثمNaOHبـز؛µؤخïضتµؤء؟إ¨¶ب£¬ذ´³ِ¼ئثم¹³ج،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
خھءث·ہضخ»·¾³خغب¾²¢¶شخ²ئّ½ّذذ×غ؛دہûسأ£¬ؤ³ءٍثل³§سأ°±ث®خüتصخ²ئّضذµؤSO2£¬شظدٍخüتصز؛ضذ¼سبëإ¨ءٍثل£¬زش»طتص¸كإ¨¶بµؤSO2²¢µأµ½¸±²ْئ·»¯·ت(NH4)2SO4؛حNH4HSO4،£خھ²â¶¨ةدتِ(NH4)2SO4؛حNH4HSO4¹ججه»ى؛دخïµؤ×é³ة£¬دض³ئب،¸أرùئ·ثؤ·ف£¬·ض±ً¼سبëدàح¬إ¨¶بµؤNaOHبـز؛¸÷40.00mL£¬¼سببضء120،و×َسز£¬ت¹°±ئّب«²؟زف³ِ£غزرضھ£؛(NH4)2SO4؛حNH4HSO4µؤ·ض½âخآ¶ب¾ù¸كسع200،و£ف£¬²âµأسذ¹طتµرéت¾فبçدآ£¨±ê×¼×´؟ِ£©£؛
| تµرéذٍ؛إ | رùئ·µؤضتء؟/g | NaOHبـز؛µؤجه»/mL | °±ئّµؤجه»/L |
| ¢ٌ | 7.4 | 40.00 | 1.68 |
| ¢ٍ | 14.8 | 40.00 | 3.36 |
| ¢َ | 22.2 | 40.00 | 1.12 |
| ¢ô | 37.0 | 40.00 | 0 |
(1) ²â¶¨¹³جضذسذ¹ط·´س¦µؤہë×س·½³جت½خھ
______________________________ ،£
(2) 3.7g¸أرùئ·½ّذذح¬رùتµرéت±£¬ةْ³ةµؤ°±ئّشع±ê×¼×´؟ِدآجه»خھ__________L،£
(3) تشاَثم¸أ»ى؛دخïضذ(NH4)2SO4؛حNH4HSO4µؤخïضتµؤء؟ض®±ب،£
(4) سû¼ئثم¸أNaOHبـز؛µؤخïضتµؤء؟إ¨¶بس¦ر،شٌµع______×éت¾ف£¬²¢سة´ث¼ئثمNaOHبـز؛µؤخïضتµؤء؟إ¨¶ب£¬ذ´³ِ¼ئثم¹³ج،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
خھءث·ہضخ»·¾³خغب¾²¢¶شخ²ئّ½ّذذ×غ؛دہûسأ£¬ؤ³ءٍثل³§سأ°±ث®خüتصخ²ئّضذµؤSO2£¬شظدٍخüتصز؛ضذ¼سبëإ¨ءٍثل£¬زش»طتص¸كإ¨¶بµؤSO2²¢µأµ½¸±²ْئ·»¯·ت(NH4)2SO4؛حNH4HSO4،£خھ²â¶¨ةدتِ(NH4)2SO4؛حNH4HSO4¹ججه»ى؛دخïµؤ×é³ة£¬دض³ئب،¸أرùئ·ثؤ·ف£¬·ض±ً¼سبëدàح¬إ¨¶بµؤNaOHبـز؛¸÷40.00mL£¬¼سببضء120،و×َسز£¬ت¹°±ئّب«²؟زف³ِ£غزرضھ£؛(NH4)2SO4؛حNH4HSO4µؤ·ض½âخآ¶ب¾ù¸كسع200،و£ف£¬²âµأسذ¹طتµرéت¾فبçدآ£¨±ê×¼×´؟ِ£©£؛
| تµرéذٍ؛إ | رùئ·µؤضتء؟/g | NaOHبـز؛µؤجه»/mL | °±ئّµؤجه»/L |
| ¢ٌ | 7.4 | 40.00 | 1.68 |
| ¢ٍ | 14.8 | 40.00 | 3.36 |
| ¢َ | 22.2 | 40.00 | 1.12 |
| ¢ô | 37.0 | 40.00 | 0 |
£¨1£©²â¶¨¹³جضذسذ¹ط·´س¦µؤہë×س·½³جت½خھ
_____________________________ ،£
£¨2£©3.7g¸أرùئ·½ّذذح¬رùتµرéت±£¬ةْ³ةµؤ°±ئّشع±ê×¼×´؟ِدآجه»خھ__________L،£
£¨3£©تشاَثم¸أ»ى؛دخïضذ(NH4)2SO4؛حNH4HSO4µؤخïضتµؤء؟ض®±ب،£
£¨4£©سû¼ئثم¸أNaOHبـز؛µؤخïضتµؤء؟إ¨¶بس¦ر،شٌµع______×éت¾ف£¬²¢سة´ث¼ئثمNaOHبـز؛µؤخïضتµؤء؟إ¨¶ب£¬ذ´³ِ¼ئثم¹³ج،£
²é؟´´ً°¸؛ح½âخِ>>
°ظ¶بضآذإ - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com