
 Ė®µÄµēĄė³Ģ¶ČÓėČÜŅŗÖŠĖłČܽāµÄµē½āÖŹÓŠ¹Ų£¬ČēĶ¼ŹĒÓĆŅ»¶ØÅØ¶ČµÄŃĪĖįµĪ¶ØV mLĶ¬ÅØ¶ČµÄNH3•H2OŹ±µĆµ½µÄµĪ¶ØĒśĻߣ®
Ė®µÄµēĄė³Ģ¶ČÓėČÜŅŗÖŠĖłČܽāµÄµē½āÖŹÓŠ¹Ų£¬ČēĶ¼ŹĒÓĆŅ»¶ØÅØ¶ČµÄŃĪĖįµĪ¶ØV mLĶ¬ÅØ¶ČµÄNH3•H2OŹ±µĆµ½µÄµĪ¶ØĒśĻߣ®·ÖĪö £Ø1£©ŃĪĖįµĪ¶ØV mLĶ¬ÅØ¶ČµÄNH3•H2OŹ±ČÜŅŗÖŠÓŠļ§øłĄė×Ó”¢ĒāĄė×Ó”¢ĀČĄė×ÓŗĶĒāŃõøłĄė×Ó£¬øł¾ŻµēŗÉŹŲŗćc£ØCl-£©+c£ØOH-£©=£ØNH4+£©+c£ØH+£©£¬Čē¹ūc£ØCl-£©=£ØNH${\;}_{4}^{+}$£©£¬Ōņc£ØOH-£©=c£ØH+£©ČÜŅŗ³ŹÖŠŠŌ£¬NH3•H2O¶ŌĖ®µÄµēĄėĘšµÖÖĘ×÷ÓĆ£¬¶ųNH4Cl¶ŌĖ®µÄµēĄėĘš“Ł½ų×÷ÓĆ£¬ÓÉ“Ė·ÖĪö½ā“š£»
£Ø2£©·Ö±šŠ“³öČēĶ¼ÖŠæÉÖŖaŹĒ°±Ė®ŗĶĀČ»Æļ§µÄ»ģŗĻĪļ”¢dŃĪĖįŗĶĀČ»Æļ§µÄ»ģŗĻĪļ£¬ÓÉ“Ė·ÖĪö½ā“š£®
½ā“š £Ø1£©ŃĪĖįµĪ¶ØV mLĶ¬ÅØ¶ČµÄNH3•H2OŹ±ČÜŅŗÖŠÓŠļ§øłĄė×Ó”¢ĒāĄė×Ó”¢ĀČĄė×ÓŗĶĒāŃõøłĄė×Ó£¬øł¾ŻµēŗÉŹŲŗćc£ØCl-£©+c£ØOH-£©=£ØNH4+£©+c£ØH+£©£¬Čē¹ūc£ØCl-£©=£ØNH${\;}_{4}^{+}$£©£¬Ōņc£ØOH-£©=c£ØH+£©ČÜŅŗ³ŹÖŠŠŌ£¬¼“ĪŖbµć£¬NH3•H2O¶ŌĖ®µÄµēĄėĘšµÖÖĘ×÷ÓĆ£¬¶ųNH4Cl¶ŌĖ®µÄµēĄėĘš“Ł½ų×÷ÓĆ£¬ĖłŅŌĮ½ÕßĒ”ŗĆĶźČ«·“Ӧɜ³ÉĀČ»Æļ§£¬¶ŌĖ®µēĄė“Ł½ų³Ģ¶Č×ī“󣬼“ĪŖc£¬¹Ź“š°øĪŖ£ŗb£»c£»Į½ÕßĒ”ŗĆĶźČ«·“Ӧɜ³ÉĀČ»Æļ§£¬¶ŌĖ®µēĄė“Ł½ų³Ģ¶Č×ī“ó£»
£Ø2£©·Ö±šŠ“³öČēĶ¼ÖŠæÉÖŖaŹĒ°±Ė®ŗĶĀČ»Æļ§µÄ»ģŗĻĪļ£¬»ģŗĻĪļČÜŅŗÖŠ°±Ė®µÄµēĄėĪŖÖ÷£¬ĖłŅŌĄė×ÓÅØ¶Č“óŠ”ĪŖ£ŗc£ØNH4+£©£¾c£ØCl-£©£¾c£ØOH-£©£¾c£ØH+£©£¬dŃĪĖįŗĶĀČ»Æļ§µÄ»ģŗĻĪļČÜŅŗ³ŹĖįŠŌ£¬ĖłŅŌĄė×ÓÅØ¶Č“óŠ”ĪŖ£ŗc£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£¬¹Ź“š°øĪŖ£ŗc£ØNH4+£©£¾c£ØCl-£©£¾c£ØOH-£©£¾c£ØH+£©£»c£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£®
µćĘĄ ±¾Ģāæ¼²éĮĖČõµē½āÖŹµÄµēĄė”¢ŃĪĄąĖ®½āµČÖŖŹ¶µć£¬øł¾ŻČõµē½āÖŹµēĄė”¢ŃĪĄąĖ®½āĢŲµćĄ“·ÖĪö½ā“š¼“æÉ£¬½įŗĻĪļĮĻŹŲŗćŗĶµēŗÉŹŲŗć½ā“š£¬øł¾ŻĄė×ÓµÄĖ®½āČ·¶ØŃōĄė×ÓÅضČĻą¶Ō“󊔣¬ÄѶČÖŠµČ£®


 ¾ŁŅ»·“ȿʌĩ°Ł·Ö³å“Ģ¾ķĻµĮŠ“š°ø
¾ŁŅ»·“ȿʌĩ°Ł·Ö³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 24gĆ¾µÄ×īĶā²ćµē×ÓŹżĪŖNA | |
| B£® | ³£ĪĀ³£Ń¹ĻĀ£¬18g H2Oŗ¬ÓŠµÄŌ×Ó×ÜŹżĪŖ3 NA | |
| C£® | ±ź×¼×“æöĻĀ£¬11.2LCH3CH2OHÖŠŗ¬ÓŠ·Ö×ӵďżÄæĪŖ0.5 NA | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ£¬2.24LCOŗĶCO2»ģŗĻĘųĢåÖŠŗ¬ÓŠµÄĢ¼Ō×ÓŹżÄæĪŖ0.1 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £©ŹĒ×ī³£ÓƵķĒēŽĢåĻūŃ×½āČČÕņĶ“Ņ©£¬æÉÓĆČēĻĀ·½·ØŗĻ³É£ŗ
£©ŹĒ×ī³£ÓƵķĒēŽĢåĻūŃ×½āČČÕņĶ“Ņ©£¬æÉÓĆČēĻĀ·½·ØŗĻ³É£ŗ

 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ¹żĀĖ | B£® |  ÉųĪö | ||
| C£® |  ŻĶČ” | D£® |  ¶”“ļ¶ūŠ§Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ba£ØOH£©2ČÜŅŗÖŠµĪ¼ÓNaHSO4ČÜŅŗÖĮÖŠŠŌ£ŗBa2++2OH-+2H++SO${\;}_{4}^{2-}$ØTBaSO4”ż+2H2O | |
| B£® | ÓĆĀČ»ÆĢśČÜŅŗŗĶ·ŠĖ®·“Ó¦ÖĘČ”ĒāŃõ»ÆĢś½ŗĢå£ŗFe3++3H2O£Ø·ŠĖ®£©ØTFe£ØOH£©3”ż+3H+ | |
| C£® | ÄĘÓėĖ®·“Ó¦£ŗNa+2H2O=Na++2OH-+H2”ü | |
| D£® | ³ĪĒåŹÆ»ŅĖ®ÓėĻ”ŃĪĖį·“Ó¦£ŗCa£ØOH£©2+2H+=Ca2++2H20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Ķ¬ĪĀĻĀ£¬µČĪļÖŹµÄĮæÅØ¶ČµÄA12£ØSO4£©3±ČMgSO4ČÜŅŗµÄpHŠ”£¬ĖµĆ÷Ć¾±ČĀĮµÄ½šŹōŠŌĒæ | |
| B£® | ĻņNa2SiO3ČÜŅŗÖŠµĪČĖ·ÓĢŖ£¬ČÜŅŗ±äŗģ£¬ŌŁµĪ¼ÓĻ”ŃĪĖį£¬ČÜŅŗŗģÉ«±äĒ³Ö±ÖĮĻūŹ§£®Ö¤Ć÷·Ē½šŹōŠŌ£ŗC1£¾Si | |
| C£® | ²ā¶ØµČĪļÖŹµÄĮæÅØ¶ČµÄNa2CO3ŗĶNa2SiO3ČÜŅŗµÄpH£¬Č·¶ØĢ¼”¢¹čĮ½ŌŖĖŲ·Ē½šŹōŠŌµÄĒæČõ | |
| D£® | 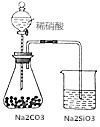 ČēĶ¼×¶ŠĪĘæÖŠÓŠĘųĢå²śÉś£¬ÉÕ±ÖŠŅŗĢå±ä»ė×Ē£¬æÉÖ¤Ć÷N”¢C”¢Si·Ē½šŹōŠŌŅĄ“Ī¼õČõ ČēĶ¼×¶ŠĪĘæÖŠÓŠĘųĢå²śÉś£¬ÉÕ±ÖŠŅŗĢå±ä»ė×Ē£¬æÉÖ¤Ć÷N”¢C”¢Si·Ē½šŹōŠŌŅĄ“Ī¼õČõ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 10molŗĶ10% | B£® | 20molŗĶ40% | C£® | 20molŗĶ20% | D£® | 30molŗĶ80% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Čōx£¾zŌņa£¾b | B£® | x=y£¾z Ōņa£¼b | C£® | x=z£¼yŌņa=b | D£® | x£¼z=yŌņa£¼b |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com