
øł¾ŻĶ¼»Ų“šĪŹĢā£ŗ
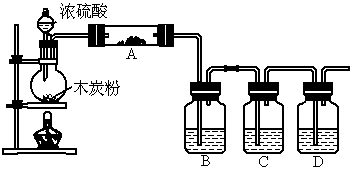
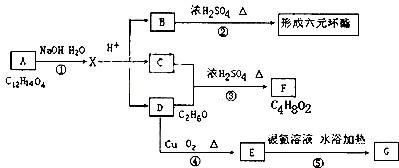
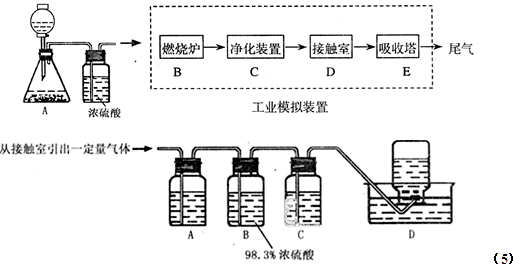
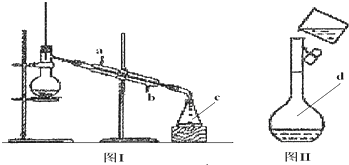
(1)ÉĻŹö×°ÖĆÖŠ£¬ŌŚ·“Ó¦Ē°ÓĆŹÖÕĘ½ōĢłÉÕĘæĶā±Ś¼ģ²é×°ÖƵÄĘųĆÜŠŌ£¬Čē¹Ū²ģ²»µ½Ć÷ĻŌµÄĻÖĻ󣬻¹æÉŅŌÓĆŹ²Ć“¼ņµ„µÄ·½·ØÖ¤Ć÷øĆ×°ÖĆ²»Ā©Ęų£®
___________________________________________________________£®
(2)Š“³öÅØĮņĖįŗĶľĢæ·ŪŌŚ¼ÓČČĢõ¼žĻĀ·¢Éś·“Ó¦µÄ»Æѧ³ĢŹ½£ŗ
____________________________________________________________£®
(3)Čē¹ūÓĆĶ¼ÖŠµÄ×°ÖĆ¼ģŃéÉĻŹö·“Ó¦µÄČ«²æ²śĪļ£¬Š“³öĻĀĆ걟ŗÅĖł±ķŹ¾µÄŅĒĘ÷ÖŠÓ¦¼ÓČėµÄŹŌ¼ĮµÄĆū³Ę¼°Ęä×÷ÓĆ£®
AÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ____________£¬×÷ÓĆŹĒ__________________________£®
BÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ____________£¬×÷ÓĆŹĒ__________________________£®
CÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ___________£¬×÷ÓĆŹĒ³ż¾”___________________ĘųĢ壮
DÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ______________£¬×÷ÓĆŹĒ_______________________£®
(4)ŹµŃ鏱£¬CÖŠÓ¦¹Ū²ģµ½µÄĻóŹĒ_________________________________£®
 ĆūŠ£Į·æ¼¾ķĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
ĆūŠ£Į·æ¼¾ķĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

 +2NaOH
+2NaOH| ”÷ |
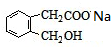 +CH3COONa+CH3CH2OH
+CH3COONa+CH3CH2OH +2NaOH
+2NaOH| ”÷ |
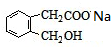 +CH3COONa+CH3CH2OH
+CH3COONa+CH3CH2OH

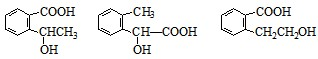 ÖŠČĪŅāŅ»ÖÖ
ÖŠČĪŅāŅ»ÖÖ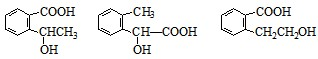 ÖŠČĪŅāŅ»ÖÖ
ÖŠČĪŅāŅ»ÖÖ| “߻ƼĮ |
 £Ø»ņCH2=CH2+H2O
£Ø»ņCH2=CH2+H2O| “߻ƼĮ |
| “߻ƼĮ |
 £Ø»ņCH2=CH2+H2O
£Ø»ņCH2=CH2+H2O| “߻ƼĮ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


| ÅØĮņĖį |
| ”÷ |
| ÅØĮņĖį |
| ”÷ |
| ”÷ |
| ”÷ |
 £Ø»ņ
£Ø»ņ ”¢
”¢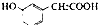 £©
£© £Ø»ņ
£Ø»ņ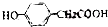 ”¢
”¢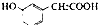 £©
£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 øł¾ŻĶ¼»Ų“šĪŹĢā£®
øł¾ŻĶ¼»Ų“šĪŹĢā£®
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
| ||
| ||
| Ń¹Ēæ/Mpa ×Ŗ»ÆĀŹ ĪĀ¶Č/”ę |
0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com