
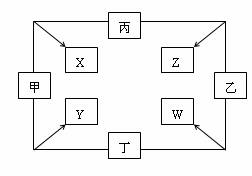
�ס��ҡ����������ֵ����ڵ�ȼ�����·�Ӧ���ɷֱ�����X��Y��Z��W���ֻ������ת����ϵ��ͼ��ʾ����֪���ټס��ҡ���Ϊ������Ԫ�صĵ��ʣ�ͨ��״����Ϊ���壻���������г�����������ͨ��״���£�X����ɫҺ�壬Y�Ǻ�ɫ���塣�۱�������ȼ�շ�����ɫ���档
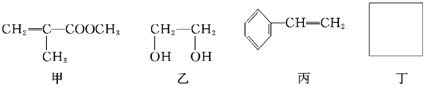
��1�����ȵĶ����ҷ�Ӧ������Ϊ �������Ķ���W��Ӧ�����ӷ���ʽΪ
��
��2��Y��Z����Һ��Ӧ�����ӷ���ʽΪ ���Ƚ�Z���ҵ�������ǿ����ʵ����ʵΪ ��
��3��������W�ı�����Һ������ڵ�X�У��õ����ɫҺ�壬��Ӧ�����ӷ���ʽΪ
��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
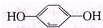
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | ��Ϻ���Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | C1 | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��8 |
| �� | 0.1 | 0.1 | pH=9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ϡH2SO4 |
| �� |
| һ������ |
| �Լ�X |
| NaOH��Һ |
| һ�������� |
| һ�������� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2010��2011ѧ���һ��ѧ����ĩ���Ի�ѧ���� ���ͣ�022
����ͼ���ס��ҡ����������ֵ����ڵ�ȼ�����·�Ӧ����X��Y��Z��W���ֻ������֪���ס��ҡ����ڳ�����Ϊ���壬����һ�ֻ��ý�������������ȼ�շ�����ɫ���棬���ڼ���ȼ�շ�����ɫ���棬Y�ǵ���ɫ���壮����գ�

(1)д����ѧʽ����________����________
(2)д����ѧ����ʽ�����õ����ű������ת�Ƶķ������Ŀ��X��Y��________
(3)д�����ӷ���ʽ��X������________
(4)����W����ɣ����������ӵķ�����________�����������ӵķ����ǽ�W����ˮ������________�Լ���
(5)��������Z���Ƿ�����ң�������________���������________����˵��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ���ס��ҡ����������ֵ����ڵ�ȼ�����·�Ӧ����X��Y��Z��W���ֻ������֪���ס��ҡ����ڳ�����Ϊ���壬����һ�ֻ��ý�������������ȼ�շ�����ɫ���棬���ڼ���ȼ�շ�����ɫ���棬Y�ǵ���ɫ���塣����գ�
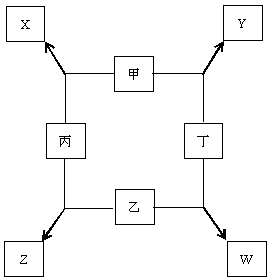
��1��д����ѧʽ����___________����___________
��2��д����ѧ����ʽ�����õ����ű������ת�Ƶķ������Ŀ��X��Y��_____________________
��3��д�����ӷ���ʽ��X������__________________
��4������W����ɣ����������ӵķ�����_________�����������ӵķ����ǽ�W����ˮ������___________�Լ���
��5����������Z���Ƿ�����ң�������___________��������� ����˵��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com