
��֪A��B��c��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣Bԭ�ӵ�L��p�������5�����ӣ�C�����ڱ���1-18���еĵ�14��Ԫ�أ�D��E��ͬһ���ڣ���֪Eԭ�ӵ�L�������������������֮��Ϊ4��1����d����еĵ�����������������֮��Ϊ5��1��D��B���γ����ӻ�����侧���ṹ����ͼ����ش�
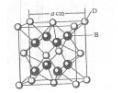
��1��A��c�γɵĹ��ۻ�����ķ���ʽ�� ���ӻ������____�����ӵ�����ṹ��____��
��2��B��C�Ƚϣ��縺�Խ�С���� ����Ԫ�ط��ţ���B��c�γɵĻ����ᄃ�������� ��
��3��E��Ԫ�����ڱ��е� ���ڣ��� ���Ԫ�أ���Ԫ�������� ��
����+2�����ӵĵ����Ų�ʽΪ ��
��4����ͼ�п��Կ�����D��B�γɵ����ӻ�����Ļ�ѧʽΪ ������þ����ı߳�Ϊa cm��������ӻ����ᄃ����ܶ��� ��ֻҪ���г���ʽ����
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
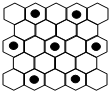 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ20�ŵ�Ԫ�أ����ǵ�ԭ��������������Aԭ�ӵļ۵��Ӳ��p�����ֻ��1�����ӣ�B��C��DԪ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ�����B��DԪ�ص�ԭ�ӵ�p�ܼ��϶���1��δ�ɶԵ��ӣ�Dԭ�ӵ�һ����������3p�����3p����ѳ�����Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�E��ͬ���ڵ�һ��������С��Ԫ�أ��ش��������⣺
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ20�ŵ�Ԫ�أ����ǵ�ԭ��������������Aԭ�ӵļ۵��Ӳ��p�����ֻ��1�����ӣ�B��C��DԪ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ�����B��DԪ�ص�ԭ�ӵ�p�ܼ��϶���1��δ�ɶԵ��ӣ�Dԭ�ӵ�һ����������3p�����3p����ѳ�����Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�E��ͬ���ڵ�һ��������С��Ԫ�أ��ش��������⣺

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
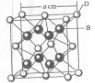
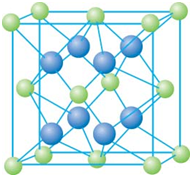 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�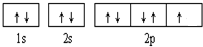


| 4��78g/mol |
| ag/cm3��6.02��1023/mol |
| 4��78g/mol |
| ag/cm3��6.02��1023/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
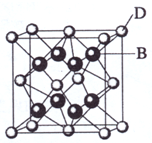 ��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��
��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?����һģ��[��ѧ--ѡ�����ʽṹ������]
��2011?����һģ��[��ѧ--ѡ�����ʽṹ������]| 4��78/mol |
| a3cm3?6.02��1023mol-1 |
| 4��78/mol |
| a3cm3?6.02��1023mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com