
ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L�� ����Һ�����к��ȵIJⶨ��
����Һ�����к��ȵIJⶨ��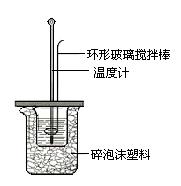
������0.50mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH���� g��
��2����������NaOH����IJ������̣�
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ/mol����
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/��[��Դ:ѧ����ZXXK] | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
��1��5.0 ��2�֣�
��2����3�֣���ȡһ�ྻ����С�ձ�����Ϊag
������ƽ�����������벢�ƶ����룬ʹ���롢�������Ϊ(a+5)g
����ҩ���������ձ��л�������NaOH������ƽǡ��ƽ�⼴�ɡ�
����������ˣ���������ȷ����Ӱ��÷֣�
��1�� (3��)
(3��)
��ϵ��������Ӧ�ġ�HҲ����,ͬ�����֣�ûע����aq�������֣�
��2���� 4.0��д��4�������֣���2�֣�
�� -53.5 kJ/mol ��û��-���Ż�û��λ�������֣���2�֣�
�� a c d (ѡ��һ����1��)��3�֣�
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��
������0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH���� g��
��2����ͼ1��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ���� ��
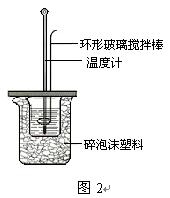
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ2��ʾ��
��3��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ/mol����
��4��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�ڽ�����Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c=4.18 J/(g����)�����к��ȡ�H= ��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ�� ��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ����������������һ�и߶���һ���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��12�֣�ijʵ��С����0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ�����к��ȵIJⶨ��
������0.50 mol��L-1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH���� g��
��2������ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ���� ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ������ͼ��ʾ��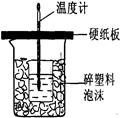
���к��ȵIJⶨ��
��3����ʵ�����������ͼ����ȱ�ٵ����ֲ��������� __��________��
��4��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�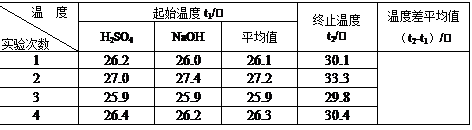
�ڽ�����Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c =" 4.18" J/(g����)�����к��ȡ�H= ��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ�� ��
a��ʵ��װ�ñ��¡�����Ч����
b������0.50 mol/L NaOH��Һʱ���ӿ̶��߶���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
e������Ͳ��ȡNaOH��Һ�����ʱ���Ӷ���
��5��ʵ���и���30 mL 0.50 mol/L�������50mL 0.55 mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �����ȡ�����ȡ����������к��ȵ���ֵ�� __________�����ȡ�����ȡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�캣��ʡ�����и߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L H2SO4��Һ�����к��ȵIJⶨ��
��.����0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ����������Ҫ����NaOH���� g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(�����) ��
|
���� |
������ƽ(������) |
С�ձ� |
����ǯ |
������ |
ҩ�� |
��Ͳ |
|
���� |
|
|
|
|
|
|
|
��� |
a |
b |
c |
d |
e |
f |
��.�ⶨ�к��ȵ�ʵ��װ����ͼ��ʾ

��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3 kJ��mol��1)��_______________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL�������ʵ�飬ʵ���������±���
������д�±��еĿհף�
|
�¶� ʵ����� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
ƽ���¶Ȳ� (t2��t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
30.1 |
|
|
2 |
27.0 |
27.4 |
27.2 |
33.3 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
�ڸ�������ʵ�����ݼ�������к���Ϊ53.5 kJ/mol�������к��ȵ�����ֵ57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)______��
a��ʵ��װ�ñ��¡�����Ч����
b������ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ������ѧ�ڵ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ������
ijʵ��С����0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ�����к��ȵIJⶨ��
������0.50 mol��L-1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH�������� ��g��
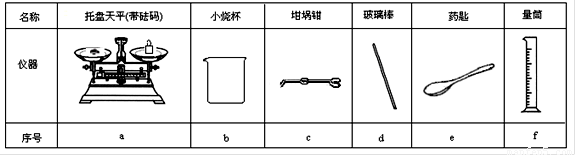
��2������ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ������ ������
|
���� |
������ƽ (������) |
С�ձ� |
����ǯ |
������ |
ҩ�� |
��Ͳ |
|
���� |
|
|
|
|
|
|
|
��� |
a |
b |
c |
d |
e |
f |
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
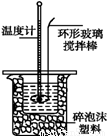
��1��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ�����к���Ϊ57.3 kJ��mol-1�� ���� ���� ���� ���� ��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
|
�¶� ʵ������� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
�¶Ȳ� ƽ��ֵ (t2-t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
29.6 |
|
|
2 |
27.0 |
27.4 |
27.2 |
31.2 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
|
���ϱ��е��¶Ȳ�ƽ��ֵΪ�� ��
�ڽ�����Ϊ0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ���ܶȶ���1 g��cm-3���кͺ�������Һ�ı�����c=4.18 J��(g����)-1�����к��Ȧ�H=������ ��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ��mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ������������
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������и߶���һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣�ijʵ��С����0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ�����к��ȵIJⶨ��
��������0.50 mol��L-1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH���� g��
��2������ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ���� ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ������ͼ��ʾ��
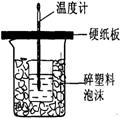
�����к��ȵIJⶨ��
��3����ʵ�����������ͼ����ȱ�ٵ����ֲ��������� __��________��
��4��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
�� ����д�±��еĿհף�
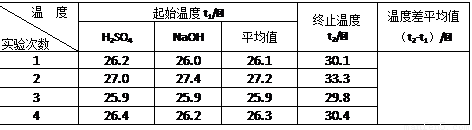
�� ������Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c = 4.18 J/(g����)�����к��ȡ�H = ��ȡС�����һλ����
�� ����ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ�� ��
a��ʵ��װ�ñ��¡�����Ч����
b������0.50 mol/L NaOH��Һʱ���ӿ̶��߶���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
e������Ͳ��ȡNaOH��Һ�����ʱ���Ӷ���
��5��ʵ���и���30 mL 0.50 mol/L�������50mL 0.55 mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �����ȡ�����ȡ����������к��ȵ���ֵ�� __________�����ȡ�����ȡ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com