
ʵ����Ҫ����1.84 mol����-1��ϡ����500mL���ش��������⣺
��1����Ҫ98%�ܶ�Ϊ1.84 g��cm-3��Ũ���� mL
��2������ʱ������ʹ�õ������� (�����)
���ձ�����50 mL��Ͳ������100 mL��Ͳ ��1000 mL����ƿ ��500 mL����ƿ
��������ƽ(������) �߲����� ��ȱ�ٵ������� �� ��
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� ��
��4�����в�����˳���ǣ�����ĸ��ʾ�� ��
A. ��ȴ B.��ȡ C.ϴ������ D.���� E.ϡ�� F.ҡ�� G.ת��
��5�����в����У�����ƿ�����߱��Ĺ������� ������(�����)��
A. ����һ�����ȷŨ�ȵı���Һ B. ����������Һ
C. ���������ܽ�������� D. ��Ϊ��Ӧ����
��6�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
�� ����Ͳ��ȡŨ����ʱ�����Ӷ���
�� ת�ƺ�û��ϴ���ձ��Ͳ�����
�� ����ʱ�����Ӷ���
�� ����ʱ����������ˮ�����̶��ߺ������������Һ
��1��50
��2���٢ڢݢߣ���ͷ�ι�
��3�����衢����
��4��BEAGCGDF��
��5��BCD
��6����ƫ�ߢ�ƫ�͢�ƫ�ߢ�ƫ��
��������
�����������1��Ũ��������ʵ���Ũ��Ϊ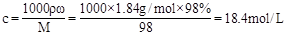 ��Ҫ����1.84 mol����-1��ϡ����500mL����Ҫ��Ũ�ȵ�Ũ����ΪV=
��Ҫ����1.84 mol����-1��ϡ����500mL����Ҫ��Ũ�ȵ�Ũ����ΪV= ��
��
��2������ʱ����ʹ�õ��������ձ���50 mL��Ͳ��500 mL����ƿ��������������֮���Ҫ��ͷ�ιܡ�
��3������ʱ�����õ������������ò�ͬ���ֱ�Ϊ�����������
��4������һ�����ʵ���Ũ����Һ�Ĺ���Ϊ�����㡢��ȡ��ϡ�͡���ȴת�ơ�ϴ�ӡ����ݡ�ҡ�Ⱥ�װƿ��ǩ��������ȷ��˳��ΪBEAGCGDF��
��5������ƿֻ��������Һ��������������Һ���ܽ�������Ϊ��Ӧ��������������ƿ�Ĺ��ܡ�����ѡBCD��
��6������c=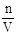 ������Ͳ��ȡŨ�������Ӷ��������ʵ����ʵ���������ֵ����Һ��Ũ��ƫ�ߣ�ת�ƺ�û��ϴ���ձ��Ͳ����������ʵ����ʵ���������ֵС����Һ��Ũ��ƫ�ͣ�����ʱ���Ӷ�������Һ�����������ֵС����Һ��Ũ��ƫ�ߣ�����ʱ��������ˮ�����̶��ߣ���Һ�����������ֵ����Һ��Ũ��ƫ�͡�
������Ͳ��ȡŨ�������Ӷ��������ʵ����ʵ���������ֵ����Һ��Ũ��ƫ�ߣ�ת�ƺ�û��ϴ���ձ��Ͳ����������ʵ����ʵ���������ֵС����Һ��Ũ��ƫ�ͣ�����ʱ���Ӷ�������Һ�����������ֵС����Һ��Ũ��ƫ�ߣ�����ʱ��������ˮ�����̶��ߣ���Һ�����������ֵ����Һ��Ũ��ƫ�͡�
���㣺һ�����ʵ���Ũ����Һ������
����������Ƚϻ�������Ҫ����ѧ���Ļ���ʵ�������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���㽭ʡ��������������ѧ2011�������ѧ�ڿ�ѧ���Ի�ѧ���� ���ͣ�022
ʵ����Ҫ����1 mol/L��ϡ����250 mL���ش��������⣺
(1)��Ҫ98���ܶ�Ϊ1.84 g/cm3��Ũ����________mL
(2)����ʱ������ʹ�õ�������________(�����)
���ձ�
��100 mL��Ͳ
��20 mL��Ͳ
��1000 mL����ƿ
��250 mL����ƿ
��������ƽ(������)
�߲���������ȱ�ٵ�������________��
(3)����ʱ����ʵ�������õ��������������÷ֱ���________��________��
(4)���ƹ����г��������������������ҺŨ���к�Ӱ��(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
��û��ϴ���ձ��Ͳ�������________��
�������ˮ�����˿̶��ߣ�ȡ��ˮʹҺ��ǡ�õ��̶��ߣ�________��
������ƿû�и��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������һ�и�һ10���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣�I��ʵ������Na2CO3��10H2O��������50 g��������Ϊ21.2����Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡNa2CO3��10H2O���� g��
��2����������ƽ��С�ձ��Ƴ�̼���ƾ������������ƽƽ����״̬����ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ����һ�������� ��ʵ�ʳ�����̼���ƾ�������Ϊ g��
II. ʵ����Ҫ����2.5 mol/L ��ϡ������Һ90 mL���ش��������⣺
��1������Ͳ��ȡ��������Ϊ98�����ܶ�Ϊ1.84 g/cm3��Ũ���� mL��
��2������ʱ������ʹ�õ���������Ͳ���ձ����������⣬��ȱ�ٵ������� ��
��3��������Һ�Ĺ����У�������������ȷ�����в�����ʹ������ҺŨ��ƫ�ߵ��� ��
| A����ȡŨ����ʱ�����Ӷ��� |
| B��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ�� |
| C��ϡ������ʱ������Һ���������� |
| D��û��ϴ��ϡ��������ձ��Ͳ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�ij��ж�����������9��ģ����У��ģ���ѧ�Ծ����������� ���ͣ�ʵ����
ʵ����Ҫ����1.84 mol����-1��ϡ����500mL���ش��������⣺
��1����Ҫ98%�ܶ�Ϊ1.84 g��cm-3��Ũ���� mL
��2������ʱ������ʹ�õ������� (�����)
���ձ�����50 mL��Ͳ������100 mL��Ͳ ��1000 mL����ƿ ��500 mL����ƿ
��������ƽ(������) �߲����� ��ȱ�ٵ������� �� ��
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� ��
��4�����в�����˳���ǣ�����ĸ��ʾ�� ��
A. ��ȴ B.��ȡ C.ϴ������ D.���� E.ϡ�� F.ҡ�� G.ת��
��5�����в����У�����ƿ�����߱��Ĺ������� ������(�����)��
A. ����һ�����ȷŨ�ȵı���Һ B. ����������Һ
C. ���������ܽ�������� D. ��Ϊ��Ӧ����
��6�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
�� ����Ͳ��ȡŨ����ʱ�����Ӷ���
�� ת�ƺ�û��ϴ���ձ��Ͳ�����
�� ����ʱ�����Ӷ���
�� ����ʱ����������ˮ�����̶��ߺ������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������и�һ10���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣�I��ʵ������Na2CO3��10H2O��������50 g ��������Ϊ21.2����Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡNa2CO3��10H2O���� g��
��2����������ƽ��С�ձ��Ƴ�̼���ƾ������������ƽƽ����״̬����ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ����һ�������� ��ʵ�ʳ�����̼���ƾ�������Ϊ g��

II. ʵ����Ҫ����2.5 mol/L ��ϡ������Һ90 mL���ش��������⣺
��1������Ͳ��ȡ��������Ϊ98�����ܶ�Ϊ1.84 g/cm3��Ũ���� mL��
��2������ʱ������ʹ�õ���������Ͳ���ձ����������⣬��ȱ�ٵ������� ��
��3��������Һ�Ĺ����У�������������ȷ�����в�����ʹ������ҺŨ��ƫ�ߵ��� ��
A����ȡŨ����ʱ�����Ӷ���
B��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ��
C��ϡ������ʱ������Һ����������
D��û��ϴ��ϡ��������ձ��Ͳ�����
E������ҡ�Ⱥ���Һ����ڱ��ߣ����ý�ͷ�ιܼ�����ˮ������
F������ƿ������
��4��������ƿ��ȡ����Һ40 mL����5 mol/L��NaOH��Һ mLǡ����ȫ��Ӧ����Ӧ����Һ�е�c(Na��)�� ��������Һ��Ϲ����е�����仯��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com