
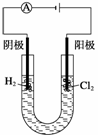
�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ���£�
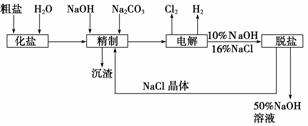
������ͼ�����������գ�
(1)�ڵ������У����Դ���������ĵ缫����������Ӧ�ĵ缫��ӦʽΪ________________________�����Դ���������ĵ缫��������ҺpH________(ѡ����䡱�������ߡ����½���)��
(2)��ҵʳ���к�Ca2����Mg2�������ʣ����ƹ����г�ȥ��Щ����ʱ������Ӧ�����ӷ���ʽΪ________________________________________________________________________��
________________________________________________________________________��
(3)���������SO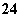 �������ߣ��������ӱ��Լ���ȥSO
�������ߣ��������ӱ��Լ���ȥSO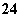 ���ñ��Լ�������________(��д��ĸ��ţ���ͬ)��
���ñ��Լ�������________(��д��ĸ��ţ���ͬ)��
A��Ba(OH)2����B��Ba(NO3)2����C��BaCl2
(4)Ϊ��Ч��ȥCa2����Mg2����SO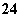 �������Լ��ĺ���˳��Ϊ________��
�������Լ��ĺ���˳��Ϊ________��
A���ȼ�NaOH�����Na2CO3���ټӱ��Լ�
B���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
C���ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��________����ȴ��________
(��д��������)��ȥNaCl��
(6)�ø�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ��������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����õ��IJ������NaClO��H2������÷�Ӧ��Ӧ�Ļ�ѧ����ʽΪ________________________________________________��
�𰸡�(1)2Cl������Cl2����2e��������
(2)Ca2����CO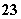 ===CaCO3��
===CaCO3��
Mg2����2OH��===Mg(OH)2��
(3)AC��(4)BC��(5)����������
(6)NaCl��H2O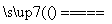 NaClO��H2��(��2NaCl��2H2O
NaClO��H2��(��2NaCl��2H2O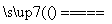 H2����Cl2����2NaOH��Cl2��2NaOH===NaCl��NaClO��H2O)
H2����Cl2����2NaOH��Cl2��2NaOH===NaCl��NaClO��H2O)
������(1)��ⱥ��ʳ��ˮ�����Դ���������ĵ缫Ϊ�������缫��Ӧʽ��2Cl������
Cl2����2e�������Դ���������ĵ缫Ϊ�������缫��Ӧʽ��2H����2e������H2��������H��������OH����������Һ��pH���ߡ�
(2)��ȥCa2����Ca2����CO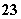 ===CaCO3������ȥMg2����Mg2����2OH��===Mg(OH)2��������Mg(OH)2���ܽ�����С��MgCO3������Mg2����CO
===CaCO3������ȥMg2����Mg2����2OH��===Mg(OH)2��������Mg(OH)2���ܽ�����С��MgCO3������Mg2����CO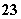 ��OH�����ʱ��һ��������Mg(OH)2������
��OH�����ʱ��һ��������Mg(OH)2������
(3)��ȥSO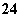 ��Ӧѡ��Ba2�����������Ba(NO3)2����Һ�л������µ����ʣ�����
��Ӧѡ��Ba2�����������Ba(NO3)2����Һ�л������µ����ʣ�����
Ba(OH)2��BaCl2���������µ����ʣ�ѡA��C��
(4)��ȥ����ʱ����Ba2����OH�������Ⱥ�֮�֣���Na2CO3һ��Ҫ�������룬��ΪCO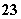 ��Ҫ��ȥ�����Ba2�������˳�������ΪNaCl���ᴿ��ѡB��C��
��Ҫ��ȥ�����Ba2�������˳�������ΪNaCl���ᴿ��ѡB��C��
(5)����ʵ�����Ƿ���NaOH��NaCl������NaCl�ܽ��С�����NaCl�����������������������������ȴ���ᾧ�������˵�NaCl���壬��Һ��һ�����Ƶ�NaOH��
(6)��2NaCl��2H2O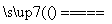 2NaOH��H2����Cl2����Cl2��2NaOH===NaCl��NaClO��H2O�ϲ������ɵó��𰸡�
2NaOH��H2����Cl2����Cl2��2NaOH===NaCl��NaClO��H2O�ϲ������ɵó��𰸡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.05 mol NaOH����ֱ���뵽100 mL����Һ���У���Һ�ĵ��������仯��С����(����)
A������ˮ
B��0.5 mol��L��1����
C��0.5 mol��L��1 CH3COOH��Һ
D��0.5 mol��L��1 KCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A����һ���¶���AgClˮ��Һ�У�Ag����Cl��Ũ�ȵij˻���һ������
B��AgCl��Ksp��1.8��10��10 mol2��L��2�����κκ�AgCl�������Һ�У�[Ag��]��[Cl��]��Ag����Cl��Ũ�ȵij˻�����1.8��10��10 mol2��L��2
C���¶�һ��ʱ������Һ��Ag����Cl��Ũ�ȵij˻�����Kspֵʱ������ҺΪAgCl�ı�����Һ
D����AgClˮ��Һ�м������ᣬKspֵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڵ��NaClˮ��Һ������������ȷ����(����)
A�����ʱ�������õ��������������õ�������
B������������������Һ�е���KI��Һ����Һ����ɫ
C������������������Һ�е����̪��Һ����Һ����ɫ
D�����һ��ʱ���ȫ�����Һת�Ƶ��ձ��У���ֽ������Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ����һU�ι���װ�뺬����ɫʯ���Na2SO4��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ�һ����������ɫ���ʺ硱����������ɫ��˳����(����)
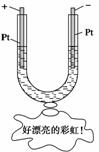
A�������ϡ��� B���졢������ C���졢�ϡ��� D���ϡ��졢��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������ˮ����ٽ������ܴ����������������(����)
A��H2PO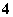 ��Na����Cl����OH��
��Na����Cl����OH��
B��Al3����Na����HCO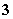 ��SO
��SO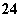
C��H����Fe2����NO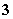 ��SO
��SO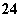
D��S2����Na����Cl����H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.02 mol��L��1 CH3COOH��Һ��0.01 mol��L��1 NaOH��Һ�Ե������ϣ���Һ�����ԣ������Һ������Ũ�ȹ�ϵ��ȷ����(����)
A��[CH3COO��]��[Na��]
B��[CH3COOH]��[CH3COO��]
C��2[H��]��[CH3COO��]��[CH3COOH]
D��[CH3COOH]��[CH3COO��]��0.02 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���20 mL 0.1 mol��L��1CH3COOH��Һ����μ���0.1 mol��L��1NaOH��Һ����pH�仯������ͼ��ʾ�����¶ȱ仯)������˵���д����������)��
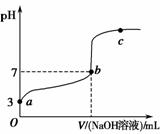
A.a��b֮�����Һ��ֻ���ڣ�cCH3COO��)>cNa��)>cH��)>cOH��)
B.b��c֮�����Һ�в����ڣ�cCH3COO��)>cH��)>cNa��)>cOH��)
C.b��ʱ��VNaOH��Һ)<20 mL����cCH3COO��)��cNa��)
D.a��b��c���ʾ����Һ��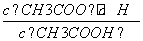 �����
�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���ɻ�ͭ��(��Ҫ�ɷ�CuFeS2)ұ��ͭ����Ҫ�������£�

(1)����A�еĴ�����Ⱦ���ѡ�������Լ��е�_________���ա�
a.Ũ���� b.ϡ����
c.NaOH��Һ d.��ˮ
(2)��ϡ�����������B��ȡ����������Һ���μ�KSCN��Һ��ʺ�ɫ��˵����Һ�д���_______(�����ӷ���)��������Һ�л�����Fe2+�ķ�����____________(ע���Լ�������)��
(3)����ͭұ����ͭ�Ļ�ѧ����ʽΪ_________________________________��
(4)��CuSO4��Һ Ϊ�������Һ���д�ͭ(��Al��Zn��Ag��Pt��Au������)�ĵ�⾫��������˵����ȷ��
Ϊ�������Һ���д�ͭ(��Al��Zn��Ag��Pt��Au������)�ĵ�⾫��������˵����ȷ�� ��__________��
��__________��
a.����ȫ��ת��Ϊ��ѧ��
b.��ͭ�ӵ�Դ����������������Ӧ
c.��Һ��Cu2+�������ƶ�
d.����������ɻ���Ag��Pt��Au�Ƚ���
(5)���÷�Ӧ2Cu+O2+2H2SO4====2CuSO4+2H2O���Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��ӦʽΪ_______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com