
¢ŁČ”Ņ»øöÅäÓŠŗĻŹŹ½ŗČūĒŅ½ą¾»”¢øÉŌļµÄ500 mL׶ŠĪĘ棬ÓĆ¾«Č·¶ČĪŖ0.001 gµÄ·ÖĪöĢģĘ½(ĻĀĶ¬)×¼Č·³ĘĮ棬µĆÖŹĮæĪŖm1”£
¢ŚĻņ׶ŠĪĘæÖŠĶØČė×ćĮæµÄøÉŌļµÄøĆ»ģŗĻĘųĢåѳʷ£¬ČūŗĆĘæČū£¬×¼Č·³ĘĮ棬µĆµ½ÖŹĮæĪŖm2”£
¢ŪĶł×¶ŠĪĘæÖŠ¼ÓĀśĖ®£¬ČūŗĆĘæČū³ĘĮ棬µĆÖŹĮæĪŖm3”£
¢ÜĖ®µÄĆܶČĪŖ¦ŃĖ® g”¤cm-3£¬æÕĘųµÄĆܶČĪŖ¦ŃæÕĘų g”¤cm-3æÕĘųµÄŹ½ĮæĪŖ29.0£¬ŌņøĆ»ģŗĻѳʷµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ£ŗ29”Į![]() ”£
ӣ
ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)ŹµŃéÄæµÄ_____________________________________________________________”£
(2)¼ĘĖć¢Ł×¶ŠĪĘæÖŠæÕĘųµÄÖŹĮ棬mæÕĘų=__________”£¢Ś×¶ŠĪĘæ֊ѳʷµÄÖŹĮæmѳʷ=_________”£
(3)ĪŹĢāĢÖĀŪ£ŗ
ĪŹĢāŅ»£ŗŹµŃéĶź³Éŗó£¬ÓŠµÄĶ¬Ń§Ģį³ö£¬ŹµŃé²»ŹĒŌŚ±ź×¼×“æöĻĀ½ųŠŠµÄ£¬Ó¦øĆ¼ĒĀ¼ŹµŃéµÄĪĀ¶Č(t ”ę)ŗĶŃ¹Ēæ(p kpa)£¬²¢¶ŌÓŠ¹ŲŹż¾Ż½ųŠŠ»»Ėć£¬ÄćČĻĪŖŹĒ·ńÓŠ±ŲŅŖ£¬²¢ĖµĆ÷ĄķÓÉ”£
“š£ŗ_________________________________________________________________”£
ĪŹĢā¶ž£ŗĄĻŹ¦¶ŌøĆŠ”×éµÄŹµŃéÉč¼ĘµćĘĄŹ±Öø³ö£ŗĪŖĮĖŹ¹ŹµŃ鏿¾ŻŗĻĄķÓŠŠ§£¬ŌŚĆæ“Ī²āĮæŹ±£¬×¶ŠĪĘæÖŠµÄĘųĢå(»ņĖ®)Ģå»ż¶¼Ó¦øĆĻąµČ”£¶Ō“Ė£¬ÄćČĻĪŖ²ÉČ”µÄ²Ł×÷ĪŖ___________________
_____________________________________________________________________ӣ
(1)²ā¶ØijĘųĢå»ģŗĻĪļѳʷµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ
![]()
(3)Ć»±ŲŅŖ£¬ŅņĪŖøĆ²ā¶ØĘųĢå»ģŗĻĪļѳʷµÄĻą¶Ō·Ö×ÓÖŹĮæĖłĄūÓƵÄŌĄķ¾ĶŹĒŌŚĶ¬ĪĀ”¢Ķ¬Ń¹”¢Ķ¬Ģå»żŹ±æÕĘųÓėѳʷµÄÖŹĮæ±ČµČÓŚĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£¬Ö»ŅŖŹĒŌŚĶ¬Ņ»ŹµŃéĢõ¼žĻĀ½ųŠŠµÄ¾ĶæÉŅŌ ŌŚµŚ1²½³ĘĮæŹ±£¬×¶ŠĪĘæ½ŗČūČū½ōŗó£¬ŌŚĘæĶā±ŚÉĻ×÷øö¼ĒŗÅ£¬ŅŌŗóĆæ“Ī³ĘĮ棬½ŗČūČūČėĘææŚµÄĪ»ÖĆŅŌ“ĖĪŖ×¼”£
½āĪö£ŗÓÉŹµŃé²½Öč¢Ł¢Ś¢ŪæɵĆѳʷ֏Į棬ÓÉ¢ÜæɵĆѳʷµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮ攣½ö“ĖŹż¾ŻŅ²Ö»ÄÜĖć³öѳʷµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮ棬Ņņ“ĖĘ䏵ŃéÄæµÄ¾ĶŹĒ²ā¶Ø»ģŗĻĘųĢåѳʷµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ


 æģ½ŻÓ¢ÓļÖÜÖÜĮ·ĻµĮŠ“š°ø
æģ½ŻÓ¢ÓļÖÜÖÜĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| Ī¢ČČ |

| ³ĮµķĪļ | Fe£ØOH£©3 | Zn£ØOH£©2 |
| pH | 1.5”«3.2 | 6.4”«8.0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğĢģ½ņŹŠŗģĒÅĒųøßČżµŚŅ»“ĪÄ£Äāæ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
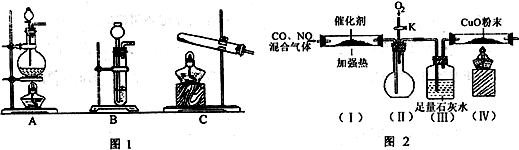
I”¢Ä³»ÆѧæĪĶā»ī¶ÆŠ”×é¶ŌĪŪČ¾“óĘųµÄ²æ·Ö·Ē½šŹōŃõ»ÆĪļ½ųŠŠĢ½¾æ”£Ēėøł¾ŻĢāÄæŅŖĒó»Ų“šĻĀĮŠĪŹĢā”£
(1)Š“³öÓĆĻõĖįÖĘČ”NOµÄĄė×Ó·½³ĢŹ½ ”£
(2)²é׏ĮĻµĆÖŖ£¬HCOOH CO+H2O”£ŹµŃéŹŅÓŠČēĶ¼lĖłŹ¾µÄ×°ÖĆ£¬ÖĘČ”COĘųĢåæÉŃ”ÓƵÄ×°ÖĆĪŖ
CO+H2O”£ŹµŃéŹŅÓŠČēĶ¼lĖłŹ¾µÄ×°ÖĆ£¬ÖĘČ”COĘųĢåæÉŃ”ÓƵÄ×°ÖĆĪŖ
(ĢīŠņŗÅ)£¬ŹµŃéŹŅĄūÓĆøĆ×°ÖĆ»¹æÉÖĘČ”µÄ³£¼ūĘųĢåÓŠ £ØŠ“Ņ»ÖÖĘųĢåµÄ·Ö×ÓŹ½£©”£
(3)²é׏ĮĻµĆÖŖ£¬ĄūÓĆ“ß»Æ¼ĮæÉŹ¹Ęū³µĪ²ĘųÖŠµÄŅ»Ńõ»ÆĢ¼ŗĶµŖŃõ»ÆĪļ“ó²æ·Ö·¢Éś·“Ó¦×Ŗ»ÆĪŖ¶žŃõ»ÆĢ¼ŗĶµŖĘų”£øĆŠ”×éŌŚŹµŃéŹŅÄ£ÄāĘū³µĪ²Ęų“¦Ąķ£¬Éč¼ĘĮĖČēĶ¼2ĖłŹ¾×°ÖĆ(²æ·Ö¼Š³ÖŗĶ×°ÖĆŅŃĀŌČ„)”£


¢ŁŹµŃéĒ°£¬¹Ų±ÕŠżČūK£¬ĻČĶصŖĘųÅž»×°ÖĆÖŠµÄæÕĘų£¬ĘäÄæµÄŹĒ ”£
¢Ś×°ÖĆ(III)µÄÖ÷ŅŖ×÷ÓĆŹĒ ”£
¢ŪøĆĢ××°ÖĆÖŠÓŠ²»ĶźÉĘÖ®“¦£¬»¹Ó¦ŌŚ×°ÖĆ(¢ō)ŗó²¹³ä ×°ÖĆ”£
II”¢øĆæĪĶāŠ”×éÉč¼ĘµÄ“ÓZnSO4”¢FeCl3µÄ»ģŗĻČÜŅŗÖŠÖĘČ”ZnSO4”¤7H2O¹ż³ĢČēĻĀ£ŗ
a£®ŌŚ»ģŗĻŅŗÖŠ¼ÓČė6 mol/L NaOHČÜŅŗ£¬ÖĮpH=8ĪŖÖ¹”£
b£®¹żĀĖŗóµĆµ½³Įµķ£¬ÓĆÕōĮóĖ®¶ą“ĪĻ“µÓ³Įµķ”£
c£®ĻņĻ“µÓŗóµÄ³ĮµķÖŠ¼ÓČė2 mol/LµÄĮņĖį£¬±£³ÖČÜŅŗµÄpHŌŚ4”«6£¬¼ÓČČÖó·Š£¬³ĆČČ¹żĀĖ£¬ĀĖŅŗ¼“ĪŖZnSO4ČÜŅŗ”£
d£®ĀĖŅŗÖŠ¼ÓČė2 mol/LµÄĮņĖį£¬Ź¹ĘäpH=2”£
ŅŃÖŖ²æ·ÖŃōĄė×ÓŅŌĒāŃõ»ÆĪļµÄŠĪŹ½æŖŹ¼³ĮµķÖĮĶźČ«³ĮµķŹ±ČÜŅŗµÄpH¼ūĻĀ±ķ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
|
³ĮµķĪļ |
Fe(OH)3 |
Zn(OH)2 |
|
pH |
1.5”«3.2 |
6.4”«8.0 |
(1)²½ÖčbÖŠČēŗĪ¼ģ²ā³ĮµķŅŃ¾Ļ“µÓøɾ» ”£
(2)²½ÖčdÖŠ¼ÓČėĮņĖį£¬Ź¹ĘäpH=2µÄÄæµÄŹĒ £»ŅŖÖʵĆZnSO4”¤7H2OµÄ²½Öčd»¹Č±ÉŁµÄ²Ł×÷ŹĒ £¬ĖłÓƵÄÖ÷ŅŖ¹čĖįŃĪŅĒĘ÷ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗģĒÅĒųŅ»Ä£ ĢāŠĶ£ŗĪŹ“šĢā
| ||
| Ī¢ČČ |
| ³ĮµķĪļ | Fe£ØOH£©3 | Zn£ØOH£©2 |
| pH | 1.5”«3.2 | 6.4”«8.0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźÉ½¶«Ź”ŹµŃé֊ѧøßæ¼»Æѧ¶žÄ£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
 CO”ü+H2O£®ŹµŃéŹŅÓŠČēĶ¼1ĖłŹ¾µÄ×°ÖĆ£¬ÖĘČ”COĘųĢåæÉŃ”ÓƵÄ×°ÖĆĪŖ______£ØĢīŠņŗÅ£©£¬ŹµŃéŹŅĄūÓĆøĆ×°ÖĆ»¹æÉÖĘČ”µÄ³£¼ūĘųĢåÓŠ______£ØĢīŠ“Ņ»ÖÖĘųĢåµÄ·Ö×ÓŹ½£©£®
CO”ü+H2O£®ŹµŃéŹŅÓŠČēĶ¼1ĖłŹ¾µÄ×°ÖĆ£¬ÖĘČ”COĘųĢåæÉŃ”ÓƵÄ×°ÖĆĪŖ______£ØĢīŠņŗÅ£©£¬ŹµŃéŹŅĄūÓĆøĆ×°ÖĆ»¹æÉÖĘČ”µÄ³£¼ūĘųĢåÓŠ______£ØĢīŠ“Ņ»ÖÖĘųĢåµÄ·Ö×ÓŹ½£©£®
| ³ĮµķĪļ | Fe£ØOH£©3 | Zn£ØOH£©2 |
| pH | 1.5”«3.2 | 6.4”«8.0 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com