
ĆŗµÄĘų»ÆŹĒĆŗµÄ×ŪŗĻĄūÓƵÄÖŲŅŖ·½·ØÖ®Ņ»£®ĆŗĘų»ÆµÄÖ÷ŅŖ»Æѧ·“Ó¦ŹĒĢæŗĶĖ®ÕōĘų·“Ӧɜ³ÉŅ»Ńõ»ÆĢ¼ŗĶĒāĘų£¬ŌŁ¾“ß»ÆŗĻ³É¼Ó¹¤³Éøß¼¶ĘūÓĶ£®Ņ»Ńõ»ÆĢ¼ŗĶĒāĘųŌŚ437”«443KĪĀ¶ČĻĀ£¬ÓĆīÜ×÷“߻ƼĮŹ±£¬æÉŅŌÉś³Én=5”«8µÄĶéĢž(ČĖŌģĘūÓĶ)£®
(1)ČōŅŌ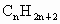 ±ķŹ¾ČĖŌģĘūÓĶ£¬ĒėŠ“³öŅ»Ńõ»ÆĢ¼ŗĶĒāĘųŗĻ³ÉČĖŌģĘūÓĶµÄ»Æѧ·½³ĢŹ½£®
±ķŹ¾ČĖŌģĘūÓĶ£¬ĒėŠ“³öŅ»Ńõ»ÆĢ¼ŗĶĒāĘųŗĻ³ÉČĖŌģĘūÓĶµÄ»Æѧ·½³ĢŹ½£®
(2)ŅŖ“ļµ½ÉĻŹöŗĻ³ÉĘūÓĶµÄŅŖĒó£¬Ņ»Ńõ»ÆĢ¼ŗĶĒāĘųµÄĢå»ż±ČµÄȔֵ·¶Ī§ŹĒ¶ąÉŁ£æ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ””°ĪåŹŠŹ®Š£”±øßČż12ŌĀĮŖŗĻ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¹ż¶ČÅÅ·ÅCO2»įŌģ³É”°ĪĀŹŅŠ§Ó¦”±£¬ĪŖĮĖ¼õÉŁĆŗČ¼ÉÕ¶Ō»·¾³Ōģ³ÉµÄĪŪČ¾£¬ĆŗµÄĘų»ÆŹĒøߊ§”¢Ēå½ąĄūÓĆĆŗĢæµÄÖŲŅŖĶ¾¾¶”£Ćŗ×ŪŗĻĄūÓƵÄŅ»ÖÖĶ¾¾¶ČēĶ¼ĖłŹ¾”£
£Ø1£©ŅŃÖŖ¢ŁC(s) £« H2O(g) = CO(g)£«H2(g) ¦¤H1£½£«131.3 kJ”¤mol£1
¢ŚC(s) £« 2H2O(g) = CO2(g) £« 2H2(g) ¦¤H2£½£«90 kJ”¤mol£1
ŌņŅ»Ńõ»ÆĢ¼ÓėĖ®ÕōĘų·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĒāĘųµÄČČ»Æѧ·½³ĢŹ½ŹĒ ________________________£¬
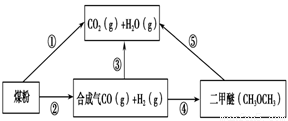
£Ø2£©ÓĆĻĀĶ¼Ōµē³Ų×°ÖĆæÉŅŌĶź³É¹ż³Ģ¢ŻµÄ×Ŗ»Æ£¬øĆ×°ÖĆbµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ_______________________”£
£Ø3£©ŌŚŃ¹ĒæĪŖ0.1 MPaĢõ¼žĻĀ£¬ČŻ»żĪŖV LµÄĆܱÕČŻĘ÷ÖŠa mol COÓė2a mol H2ŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³É¼×“¼£ŗ
CO(g)£«2H2(g)  CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ

¢Łp1________p2(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)”£
¢ŚŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ĻņČŻĘ÷ÖŠŌŁŌö¼Óa mol COÓė2a mol H2£¬“ļµ½ŠĀĘ½ŗāŹ±£¬COµÄĘ½ŗā×Ŗ»ÆĀŹ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
¢ŪŌŚp1ĻĀ£¬100 ”ꏱ£¬CO(g)£«2H2(g)
 CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
£Ø4£©ČēĶ¼±ķŹ¾CO2ÓėH2·“Ӧɜ³ÉCH3OHŗĶH2OµÄ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ”¤mol£1)µÄ±ä»Æ£ŗ
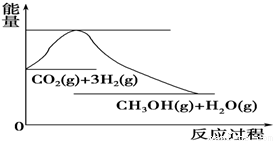
¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________(Ģī±ąŗÅ)”£
A£®¦¤H£¾0£¬¦¤S£¾0 B£®¦¤H£¾0£¬¦¤S£¼0
C£®¦¤H£¼0£¬¦¤S£¼0 D£®¦¤H£¼0£¬¦¤S£¾0
£Ø5£©ĪŖĢ½¾æ·“Ó¦ŌĄķ£¬ĻÖ½ųŠŠČēĻĀŹµŃ飬ŌŚĢå»żĪŖ1 LµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1 mol
CO2ŗĶ3 mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g)
 CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ
CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ

¢Ł“Ó·“Ó¦æŖŹ¼µ½Ę½ŗā£¬CO2µÄĘ½¾ł·“Ó¦ĖŁĀŹv(CO2)£½________”£
¢ŚĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹»ÆŃ§Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ________(Ģī±ąŗÅ)”£
A£®ÉżøßĪĀ¶Č B£®½«CH3OH(g)¼°Ź±Ņŗ»ÆŅĘ³ö
C£®Ń”Ōńøߊ§“߻ƼĮ D£®ŌŁ³äČė1 mol CO2ŗĶ3 mol H2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ””°ĪåŹŠŹ®Š£”±øßČż12ŌĀĮŖŗĻ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¹ż¶ČÅÅ·ÅCO2»įŌģ³É”°ĪĀŹŅŠ§Ó¦”±£¬ĪŖĮĖ¼õÉŁĆŗČ¼ÉÕ¶Ō»·¾³Ōģ³ÉµÄĪŪČ¾£¬ĆŗµÄĘų»ÆŹĒøߊ§”¢Ēå½ąĄūÓĆĆŗĢæµÄÖŲŅŖĶ¾¾¶”£Ćŗ×ŪŗĻĄūÓƵÄŅ»ÖÖĶ¾¾¶ČēĶ¼ĖłŹ¾”£

£Ø1£©ŅŃÖŖ¢ŁC(s) £« H2O(g) = CO(g)£«H2(g) ¦¤H1£½£«131.3 kJ”¤mol£1
¢ŚC(s) £« 2H2O(g) = CO2(g) £« 2H2(g) ¦¤H2£½£«90 kJ”¤mol£1
ŌņŅ»Ńõ»ÆĢ¼ÓėĖ®ÕōĘų·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĒāĘųµÄČČ»Æѧ·½³ĢŹ½ŹĒ ________________________£¬
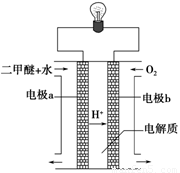
£Ø2£©ÓĆĻĀĶ¼Ōµē³Ų×°ÖĆæÉŅŌĶź³É¹ż³Ģ¢ŻµÄ×Ŗ»Æ£¬øĆ×°ÖĆbµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ_______________________”£
£Ø3£©ŌŚŃ¹ĒæĪŖ0.1 MPaĢõ¼žĻĀ£¬ČŻ»żĪŖV LµÄĆܱÕČŻĘ÷ÖŠa mol COÓė2a mol H2ŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³É¼×“¼£ŗ
CO(g)£«2H2(g)  CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ

¢Łp1________p2(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)”£
¢ŚŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ĻņČŻĘ÷ÖŠŌŁŌö¼Óa mol COÓė2a mol H2£¬“ļµ½ŠĀĘ½ŗāŹ±£¬COµÄĘ½ŗā×Ŗ»ÆĀŹ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
¢ŪŌŚp1ĻĀ£¬100 ”ꏱ£¬CO(g)£«2H2(g)
 CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
£Ø4£©ČēĶ¼±ķŹ¾CO2ÓėH2·“Ӧɜ³ÉCH3OHŗĶH2OµÄ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ”¤mol£1)µÄ±ä»Æ£ŗ

¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________(Ģī±ąŗÅ)”£
A£®¦¤H£¾0£¬¦¤S£¾0 B£®¦¤H£¾0£¬¦¤S£¼0
C£®¦¤H£¼0£¬¦¤S£¼0 D£®¦¤H£¼0£¬¦¤S£¾0
£Ø5£©ĪŖĢ½¾æ·“Ó¦ŌĄķ£¬ĻÖ½ųŠŠČēĻĀŹµŃ飬ŌŚĢå»żĪŖ1 LµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1 mol
CO2ŗĶ3 mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g)
 CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ
CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ

¢Ł“Ó·“Ó¦æŖŹ¼µ½Ę½ŗā£¬CO2µÄĘ½¾ł·“Ó¦ĖŁĀŹv(CO2)£½________”£
¢ŚĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹»ÆŃ§Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ________(Ģī±ąŗÅ)”£
A£®ÉżøßĪĀ¶Č B£®½«CH3OH(g)¼°Ź±Ņŗ»ÆŅĘ³ö
C£®Ń”Ōńøߊ§“߻ƼĮ D£®ŌŁ³äČė1 mol CO2ŗĶ3 mol H2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ””°ĪåŹŠŹ®Š£”±øßČż12ŌĀĮŖŗĻ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¹ż¶ČÅÅ·ÅCO2»įŌģ³É”°ĪĀŹŅŠ§Ó¦”±£¬ĪŖĮĖ¼õÉŁĆŗČ¼ÉÕ¶Ō»·¾³Ōģ³ÉµÄĪŪČ¾£¬ĆŗµÄĘų»ÆŹĒøߊ§”¢Ēå½ąĄūÓĆĆŗĢæµÄÖŲŅŖĶ¾¾¶”£Ćŗ×ŪŗĻĄūÓƵÄŅ»ÖÖĶ¾¾¶ČēĶ¼ĖłŹ¾”£

£Ø1£©ŅŃÖŖ¢ŁC(s) £« H2O(g) = CO(g)£«H2(g) ¦¤H1£½£«131.3 kJ”¤mol£1
¢ŚC(s) £« 2H2O(g) = CO2(g) £« 2H2(g) ¦¤H2£½£«90 kJ”¤mol£1
ŌņŅ»Ńõ»ÆĢ¼ÓėĖ®ÕōĘų·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĒāĘųµÄČČ»Æѧ·½³ĢŹ½ŹĒ ________________________£¬
£Ø2£©ÓĆĻĀĶ¼Ōµē³Ų×°ÖĆæÉŅŌĶź³É¹ż³Ģ¢ŻµÄ×Ŗ»Æ£¬øĆ×°ÖĆbµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ_______________________”£

£Ø3£©ŌŚŃ¹ĒæĪŖ0.1 MPaĢõ¼žĻĀ£¬ČŻ»żĪŖV LµÄĆܱÕČŻĘ÷ÖŠa mol COÓė2a mol H2ŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³É¼×“¼£ŗ
CO(g)£«2H2(g) 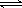 CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
¢Łp1________p2(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)”£
¢ŚŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ĻņČŻĘ÷ÖŠŌŁŌö¼Óa mol COÓė2a mol H2£¬“ļµ½ŠĀĘ½ŗāŹ±£¬COµÄĘ½ŗā×Ŗ»ÆĀŹ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
¢ŪŌŚp1ĻĀ£¬100 ”ꏱ£¬CO(g)£«2H2(g)
 CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
£Ø4£©ČēĶ¼±ķŹ¾CO2ÓėH2·“Ӧɜ³ÉCH3OHŗĶH2OµÄ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ”¤mol£1)µÄ±ä»Æ£ŗ
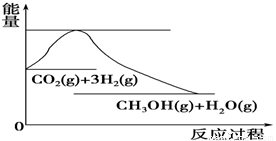
¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________(Ģī±ąŗÅ)”£
A£®¦¤H£¾0£¬¦¤S£¾0 B£®¦¤H£¾0£¬¦¤S£¼0
C£®¦¤H£¼0£¬¦¤S£¼0 D£®¦¤H£¼0£¬¦¤S£¾0
£Ø5£©ĪŖĢ½¾æ·“Ó¦ŌĄķ£¬ĻÖ½ųŠŠČēĻĀŹµŃ飬ŌŚĢå»żĪŖ1 LµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1 mol
CO2ŗĶ3 mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g)
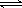 CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ
CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ
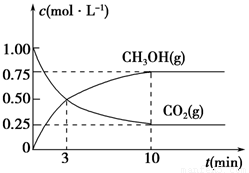
¢Ł“Ó·“Ó¦æŖŹ¼µ½Ę½ŗā£¬CO2µÄĘ½¾ł·“Ó¦ĖŁĀŹv(CO2)£½________”£
¢ŚĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹»ÆŃ§Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ________(Ģī±ąŗÅ)”£
A£®ÉżøßĪĀ¶Č B£®½«CH3OH(g)¼°Ź±Ņŗ»ÆŅĘ³ö
C£®Ń”Ōńøߊ§“߻ƼĮ D£®ŌŁ³äČė1 mol CO2ŗĶ3 mol H2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ””°ĪåŹŠŹ®Š£”±øßČż12ŌĀĮŖŗĻ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¹ż¶ČÅÅ·ÅCO2»įŌģ³É”°ĪĀŹŅŠ§Ó¦”±£¬ĪŖĮĖ¼õÉŁĆŗČ¼ÉÕ¶Ō»·¾³Ōģ³ÉµÄĪŪČ¾£¬ĆŗµÄĘų»ÆŹĒøߊ§”¢Ēå½ąĄūÓĆĆŗĢæµÄÖŲŅŖĶ¾¾¶”£Ćŗ×ŪŗĻĄūÓƵÄŅ»ÖÖĶ¾¾¶ČēĶ¼ĖłŹ¾”£
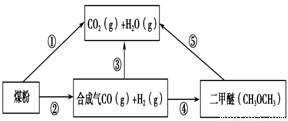
£Ø1£©ŅŃÖŖ¢ŁC(s) £« H2O(g) = CO(g)£«H2(g) ¦¤H1£½£«131.3 kJ”¤mol£1
¢ŚC(s) £« 2H2O(g) = CO2(g) £« 2H2(g) ¦¤H2£½£«90 kJ”¤mol£1
ŌņŅ»Ńõ»ÆĢ¼ÓėĖ®ÕōĘų·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĒāĘųµÄČČ»Æѧ·½³ĢŹ½ŹĒ ________________________£¬
£Ø2£©ÓĆĻĀĶ¼Ōµē³Ų×°ÖĆæÉŅŌĶź³É¹ż³Ģ¢ŻµÄ×Ŗ»Æ£¬øĆ×°ÖĆbµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ_______________________”£
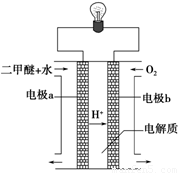
£Ø3£©ŌŚŃ¹ĒæĪŖ0.1 MPaĢõ¼žĻĀ£¬ČŻ»żĪŖV LµÄĆܱÕČŻĘ÷ÖŠa mol COÓė2a mol H2ŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³É¼×“¼£ŗ
CO(g)£«2H2(g) 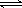 CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
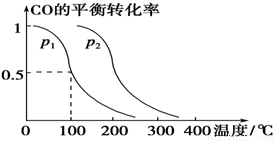
¢Łp1________p2(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)”£
¢ŚŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ĻņČŻĘ÷ÖŠŌŁŌö¼Óa mol COÓė2a mol H2£¬“ļµ½ŠĀĘ½ŗāŹ±£¬COµÄĘ½ŗā×Ŗ»ÆĀŹ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
¢ŪŌŚp1ĻĀ£¬100 ”ꏱ£¬CO(g)£«2H2(g)
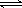 CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
£Ø4£©ČēĶ¼±ķŹ¾CO2ÓėH2·“Ӧɜ³ÉCH3OHŗĶH2OµÄ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ”¤mol£1)µÄ±ä»Æ£ŗ
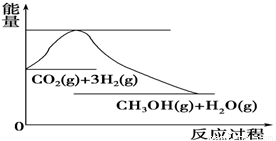
¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________(Ģī±ąŗÅ)”£
A£®¦¤H£¾0£¬¦¤S£¾0 B£®¦¤H£¾0£¬¦¤S£¼0
C£®¦¤H£¼0£¬¦¤S£¼0 D£®¦¤H£¼0£¬¦¤S£¾0
£Ø5£©ĪŖĢ½¾æ·“Ó¦ŌĄķ£¬ĻÖ½ųŠŠČēĻĀŹµŃ飬ŌŚĢå»żĪŖ1 LµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1 mol
CO2ŗĶ3 mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g)
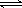 CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ
CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ
¢Ł“Ó·“Ó¦æŖŹ¼µ½Ę½ŗā£¬CO2µÄĘ½¾ł·“Ó¦ĖŁĀŹv(CO2)£½________”£
¢ŚĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹»ÆŃ§Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ________(Ģī±ąŗÅ)”£
A£®ÉżøßĪĀ¶Č B£®½«CH3OH(g)¼°Ź±Ņŗ»ÆŅĘ³ö
C£®Ń”Ōńøߊ§“߻ƼĮ D£®ŌŁ³äČė1 mol CO2ŗĶ3 mol H2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ””°ĪåŹŠŹ®Š£”±øßČż12ŌĀĮŖŗĻ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¹ż¶ČÅÅ·ÅCO2»įŌģ³É”°ĪĀŹŅŠ§Ó¦”±£¬ĪŖĮĖ¼õÉŁĆŗČ¼ÉÕ¶Ō»·¾³Ōģ³ÉµÄĪŪČ¾£¬ĆŗµÄĘų»ÆŹĒøߊ§”¢Ēå½ąĄūÓĆĆŗĢæµÄÖŲŅŖĶ¾¾¶”£Ćŗ×ŪŗĻĄūÓƵÄŅ»ÖÖĶ¾¾¶ČēĶ¼ĖłŹ¾”£

£Ø1£©ŅŃÖŖ¢ŁC(s) £« H2O(g) = CO(g)£«H2(g) ¦¤H1£½£«131.3 kJ”¤mol£1
¢ŚC(s) £« 2H2O(g) = CO2(g) £« 2H2(g) ¦¤H2£½£«90 kJ”¤mol£1
ŌņŅ»Ńõ»ÆĢ¼ÓėĖ®ÕōĘų·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĒāĘųµÄČČ»Æѧ·½³ĢŹ½ŹĒ ________________________£¬
£Ø2£©ÓĆĻĀĶ¼Ōµē³Ų×°ÖĆæÉŅŌĶź³É¹ż³Ģ¢ŻµÄ×Ŗ»Æ£¬øĆ×°ÖĆbµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ_______________________”£

£Ø3£©ŌŚŃ¹ĒæĪŖ0.1 MPaĢõ¼žĻĀ£¬ČŻ»żĪŖV LµÄĆܱÕČŻĘ÷ÖŠa mol COÓė2a mol H2ŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³É¼×“¼£ŗ
CO(g)£«2H2(g)  CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
CH3OH(g)£¬COµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬Ōņ£ŗ
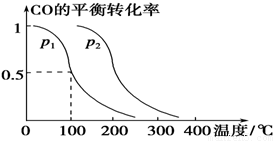
¢Łp1________p2(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)”£
¢ŚŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ĻņČŻĘ÷ÖŠŌŁŌö¼Óa mol COÓė2a mol H2£¬“ļµ½ŠĀĘ½ŗāŹ±£¬COµÄĘ½ŗā×Ŗ»ÆĀŹ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
¢ŪŌŚp1ĻĀ£¬100 ”ꏱ£¬CO(g)£«2H2(g)
 CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
CH3OH(g)·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________(ÓĆŗ¬a”¢VµÄ“śŹżŹ½±ķŹ¾)”£
£Ø4£©ČēĶ¼±ķŹ¾CO2ÓėH2·“Ӧɜ³ÉCH3OHŗĶH2OµÄ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ”¤mol£1)µÄ±ä»Æ£ŗ

¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________(Ģī±ąŗÅ)”£
A£®¦¤H£¾0£¬¦¤S£¾0 B£®¦¤H£¾0£¬¦¤S£¼0
C£®¦¤H£¼0£¬¦¤S£¼0 D£®¦¤H£¼0£¬¦¤S£¾0
£Ø5£©ĪŖĢ½¾æ·“Ó¦ŌĄķ£¬ĻÖ½ųŠŠČēĻĀŹµŃ飬ŌŚĢå»żĪŖ1 LµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1 mol
CO2ŗĶ3 mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g)
 CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ
CH3OH(g)£«H2O(g)£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗ

¢Ł“Ó·“Ó¦æŖŹ¼µ½Ę½ŗā£¬CO2µÄĘ½¾ł·“Ó¦ĖŁĀŹv(CO2)£½________”£
¢ŚĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹»ÆŃ§Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ________(Ģī±ąŗÅ)”£
A£®ÉżøßĪĀ¶Č B£®½«CH3OH(g)¼°Ź±Ņŗ»ÆŅĘ³ö
C£®Ń”Ōńøߊ§“߻ƼĮ D£®ŌŁ³äČė1 mol CO2ŗĶ3 mol H2
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com