
ŹŅĪĀĻĀ£¬½«1 molµÄCuSO4·5H2O(s)ČÜÓŚĖ®»įŹ¹ČÜŅŗĪĀ¶Č½µµĶ£¬ČČŠ§Ó¦ĪŖ¦¤H1£¬½«1 molµÄCuSO4ČÜÓŚĖ®»įŹ¹ČÜŅŗĪĀ¶ČÉżøߣ¬ČČŠ§Ó¦ĪŖ¦¤H2£»CuSO4(s)·5H2O(s)ŹÜČČ·Ö½āµÄ»Æѧ·½³ĢŹ½ĪŖCuSO4·5H2O(s)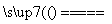 CuSO4(s)£«5H2O(l)£¬ČČŠ§Ó¦ĪŖ¦¤H3”£ŌņĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ(””””)
CuSO4(s)£«5H2O(l)£¬ČČŠ§Ó¦ĪŖ¦¤H3”£ŌņĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ(””””)
A£®¦¤H2£¾¦¤H3
B£®¦¤H1£¼¦¤H3
C£®¦¤H1£«¦¤H3£½¦¤H2
D£®¦¤H1£«¦¤H2£¾¦¤H3
“š°ø””B
½āĪö””1 mol CuSO4·5H2O(s)ČÜÓŚĖ®»įŹ¹ČÜŅŗĪĀ¶Č½µµĶ£¬ĪŖĪüČČ·“Ó¦£¬¹Ź¦¤H1£¾0,1 mol CuSO4(s)ČÜÓŚĖ®»įŹ¹ČÜŅŗĪĀ¶ČÉżøߣ¬ĪŖ·ÅČČ¹ż³Ģ£¬¹Ź¦¤H2£¼0,1 mol CuSO4·5H2O(s)ČÜÓŚĖ®æÉŅŌ·ÖĪŖĮ½øö¹ż³Ģ£¬ĻČ·Ö½ā³É1 mol CuSO4(s)ŗĶ5 molĖ®£¬Č»ŗó1 mol CuSO4(s)ŌŁČÜÓŚĖ®£¬CuSO4·5H2OµÄ·Ö½āĪŖĪüČČ·“Ó¦£¬¼“¦¤H3£¾0£¬øł¾ŻøĒĖ¹¶ØĀɵƵ½¹ŲĻµŹ½¦¤H1£½¦¤H2£«¦¤H3£¬·ÖĪöµĆµ½“š°ø£ŗ¦¤H1£¼¦¤H3”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾Ż2CrO £«2H£«??Cr2O
£«2H£«??Cr2O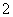 7£«H2OÉč¼ĘĶ¼Ź¾×°ÖĆ(¾łĪŖ¶čŠŌµē¼«)µē½āNa2CrO4ČÜŅŗÖĘČ”Na2Cr2O7£¬Ķ¼ÖŠÓŅ²ąµē¼«Į¬½ÓµēŌ“µÄ______¼«£¬Ęäµē¼«·“Ó¦Ź½ĪŖ_________________________”£
7£«H2OÉč¼ĘĶ¼Ź¾×°ÖĆ(¾łĪŖ¶čŠŌµē¼«)µē½āNa2CrO4ČÜŅŗÖĘČ”Na2Cr2O7£¬Ķ¼ÖŠÓŅ²ąµē¼«Į¬½ÓµēŌ“µÄ______¼«£¬Ęäµē¼«·“Ó¦Ź½ĪŖ_________________________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖAl2O3(s)£«AlCl3(g)£«3C(s)===3AlCl(g)£«3CO(g)””¦¤H£½a kJ·mol£1
ÅŠ¶ĻĻĀĮŠ±ä»Æ¹ż³ĢŹĒ·ńÕżČ·£¬ÕżČ·µÄ»®”°”Ģ”±£¬“ķĪóµÄ»®”°”Į”±
(1)3AlCl(g)£«3CO(g)===Al2O3(s)£«AlCl3(g)£«3C(s)
¦¤H£½a kJ·mol£1(””””)
(2)AlCl(g)£«CO(g)===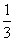 Al2O3(s)£«
Al2O3(s)£«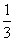 AlCl3(g)£«3C(s)
AlCl3(g)£«3C(s)
¦¤H£½£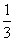 a kJ·mol£1(””””)
a kJ·mol£1(””””)
(3)2Al2O3(s)£«2AlCl3(g)£«6C(s)===6AlCl(g)£«6CO(g)
¦¤H£½£2a kJ·mol£1(””””)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖĻū³żÄæĒ°Č¼ĮĻČ¼ÉÕŹ±²śÉśµÄ»·¾³ĪŪČ¾£¬Ķ¬Ź±»ŗ½āÄÜŌ“Ī£»ś£¬ÓŠ¹Ų×ؼŅĢį³öĮĖĄūÓĆĢ«ŃōÄÜÖĘČ”ĒāÄܵĹ¹Ļė”£
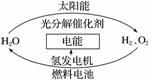
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®H2OµÄ·Ö½ā·“Ó¦ŹĒĪüČČ·“Ó¦
B£®ĒāÄÜŌ“Ņѱ»ĘÕ±éŹ¹ÓĆ
C£®2 mol ŅŗĢ¬H2O¾ßÓŠµÄ×ÜÄÜĮæµĶÓŚ2 mol H2ŗĶ1 mol O2µÄÄÜĮæ
D£®ĒāĘų²»Ņ×Öü“ęŗĶŌĖŹä£¬ĪŽæŖ·¢ĄūÓĆ¼ŪÖµ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŅ“¼ŹĒÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬æÉÓÉŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·Ø»ņ¼ä½ÓĖ®ŗĻ·ØÉś²ś”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼ä½ÓĖ®ŗĻ·ØŹĒÖøĻČ½«ŅŅĻ©ÓėÅØĮņĖį·“Ӧɜ³ÉĮņĖįĒāŅŅõ„(C2H5OSO3H)£¬ŌŁĖ®½āÉś³ÉŅŅ“¼”£Š“³öĻąÓ¦·“Ó¦µÄ»Æѧ·½³ĢŹ½_____________________________________________
________________________________________________________________________ӣ
(2)ŅŃÖŖ£ŗ
¼×“¼ĶŃĖ®·“Ó¦2CH3OH(g)===CH3OCH3(g)£«H2O(g)
¦¤H1£½£23.9 kJ·mol£1
¼×“¼ÖĘĻ©Ģž·“Ó¦2CH3OH(g)===C2H4(g)£«2H2O(g)
¦¤H2£½£29.1 kJ·mol£1
ŅŅ“¼Ņģ¹¹»Æ·“Ó¦C2H5OH(g)===CH3OCH3(g)
¦¤H3£½£«50.7 kJ·mol£1
ŌņŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·“Ó¦C2H4(g)£«H2O(g)===C2H5OH(g)µÄ¦¤H£½______________ kJ·mol£1”£
Óė¼ä½ÓĖ®ŗĻ·ØĻą±Č£¬ĘųĻąÖ±½ÓĖ®ŗĻ·ØµÄÓŵćŹĒ_____________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗ
¢ŁH2(g)£« O2(g)===H2O(g)””¦¤H1£½a kJ·mol£1
O2(g)===H2O(g)””¦¤H1£½a kJ·mol£1
¢Ś2H2(g)£«O2(g)===2H2O(g)””¦¤H2£½b kJ·mol£1
¢ŪH2(g)£« O2(g)===H2O(l)””¦¤H3£½c kJ·mol£1
O2(g)===H2O(l)””¦¤H3£½c kJ·mol£1
¢Ü2H2(g)£«O2(g)===2H2O(l)””¦¤H3£½d kJ·mol£1
ĻĀĮŠ¹ŲĻµŹ½ÖŠÕżČ·µÄŹĒ(””””)
A£®a£¼c£¼0 B£®b£¾d£¾0
C£®2a£½b£¼0 D£®2c£½d£¾0
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
pH£½5µÄH2SO4ČÜŅŗ£¬¼ÓĖ®Ļ”ŹĶµ½500±¶£¬ŌņĻ”ŹĶŗóc(SO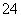 )Óėc(H£«)µÄ±ČÖµĪŖ__________”£
)Óėc(H£«)µÄ±ČÖµĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µŚĖÄÖÜĘŚÖŠ£¬Ī“³É¶Ōµē×ÓŹż×ī¶ąµÄŌŖĖŲŹĒ__________”£(ĢīĆū³Ę)
(1)ĖüĪ»ÓŚµŚ________×唣
(2)ŗĖĶāµē×ÓÅŲ¼Ź½ŹĒ________”£
(3)ĖüÓŠ________øöÄÜ²ć£¬________øöÄܼ¶£¬__________ÖÖŌĖ¶ÆדĢ¬²»Ķ¬µÄµē×Ó”£
(4)¼Ūµē×ÓÅŲ¼Ź½________£¬¼Ūµē×ÓÅŲ¼Ķ¼__________”£
(5)ŹōÓŚ________Ēų”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com