
| ʵ�� ��� | ʵ������ | ʵ���� |
| 1 | ��AgNO3��Һ | �а�ɫ�������� |
| 2 | ������NaOH��Һ������ | �ռ�������1.12L��������ɱ�״���µ������ |
| 3 | ������BaCl2��Һʱ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6.63g���ڶ��γ�������Ϊ4.66g |
| �����ӷ��� | ���ʵ���Ũ�ȣ�mol/L�� |
���� ��̼���������������Ȼ������ǰ�ɫ���������ʵ��1��ȷ���Ƿ��������Ӳ���ȷ��������ʵ��2��֪��������ӣ�����ʵ��3��֪����һ����̼�ᱵ�����ᱵ������Һ��һ������CO32-��SO42-������Һ��һ������Ba2+��Mg2+��
������NaOH��Һ�����ȣ����ռ������壬�������Ϊ�����������ݱ���°�����������Լ���笠������ʵ�����
̼�ᱵ���������ᣬ���ᱵ���������ᣬ��˼��������ʣ��4.66g����ΪBaSO4��������̼�غ㼴���������Һ��c��SO42-������CO32-�������ʵ���Ũ�ȣ�
������Ϸ����Լ����и��������ݿ�֪����Һ�п϶����ڵ�������NH4+��CO32-��SO42-�������㣬NH4+�����ʵ���Ϊ0.05 mol��CO32-��SO42-�����ʵ����ֱ�Ϊ0.01 mol��0.02 mol�����ݵ���غ��K+һ�����ڣ��Դ������
��� �⣺��1��̼���������������Ȼ������ǰ�ɫ���������ʵ��1�õ�������ȷ�����Ȼ�������ʵ��1��Cl-�Ƿ���ڵ��ж��ǣ�����ȷ����
����ʵ��2��֪��������ӣ�����ʵ��3��֪����һ����̼�ᱵ�����ᱵ������Һ��һ������CO32-��SO42-��̼�ᱵ��̼��þ�����ᱵ�ȶ��Dz�����ˮ�ij������ʿ���֪��Һһ�������ڵ������ǣ�Ba2+��Mg2+��
�ʴ�Ϊ������ȷ����Ba2+��Mg2+��
��2����ϣ�1���з�����֪��Һ��һ�����е�������ΪCO32-��SO42-����̼�ᱵ���������ᣬ���ᱵ�������������֪���������ʣ��4.66g����ΪBaSO4���������غ��֪��Һ��c��SO42-��=$\frac{\frac{4.66g}{233g/mol}}{0.1L}$=0.2mol/L��6.27g������̼�ᱵ������Ϊ6.63g-4.66g=1.97g������̼�غ��֪��Һ��c��CO32-��=$\frac{\frac{1.97g}{197g/mol}}{0.1L}$=0.1mol/L���ʴ�Ϊ��
| �����ӷ��� | ���ʵ���Ũ�ȣ�mol/L�� |
| CO32- | 0.1 |
| SO42- | 0.2 |
���� ���⿼�鳣�����ӵļ��飬Ϊ��Ƶ���㣬�������Ӽ�����Լ������������Ϊ�����Ĺؼ������ط�����ʵ�������Ŀ��飬ע�����ʵ����ļ��㼰����غ�Ӧ�ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
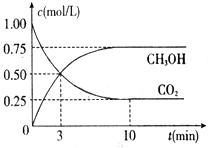
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��A-����c��R+����c��H+����c��OH-�� | B�� | c��A-����c��R+����c��OH-����c��H+�� | ||
| C�� | c��R+����c��A-����c��H+����c��OH-�� | D�� | c��R+����c��A-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
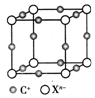 ǰ4����Ԫ��A��B��C��D��E��F��ԭ������������������A��Bͬ���ڣ���̬��AB2���������������������C��Eԭ�Ӷ���һ��δ�ɶԵ��ӣ�C+��E-��һ�����Ӳ㣬Eԭ�ӵ�һ�����Ӻ�3p���ȫ������D������ϼۺ���ͻ��ϼ۴�����Ϊ4���������������D����������Ϊ40%���Һ���������������������FΪ��ɫ���ʣ��㷺���ڵ�����ҵ���ش��������⣺
ǰ4����Ԫ��A��B��C��D��E��F��ԭ������������������A��Bͬ���ڣ���̬��AB2���������������������C��Eԭ�Ӷ���һ��δ�ɶԵ��ӣ�C+��E-��һ�����Ӳ㣬Eԭ�ӵ�һ�����Ӻ�3p���ȫ������D������ϼۺ���ͻ��ϼ۴�����Ϊ4���������������D����������Ϊ40%���Һ���������������������FΪ��ɫ���ʣ��㷺���ڵ�����ҵ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
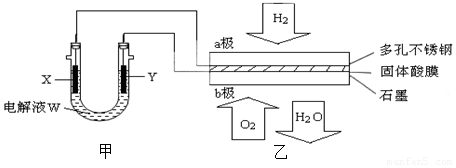
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 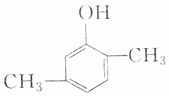 �� ��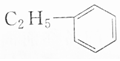 | B�� |  �� ��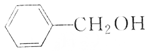 | ||
| C�� | 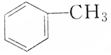 �� �� | D�� | CH3CH2OH��CH2OH-CH2OH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������Ϊ2��3-���һ�-1-��ϩ��
������Ϊ2��3-���һ�-1-��ϩ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com