
��֪����![]()
��R��CH2��CH��CH2��Cl2![]() R��CHCl��CH��CH2��HCl
R��CHCl��CH��CH2��HCl
�л���M�ڹ�ҵ�Ͽ������ܼ�����ϳ����������̣�����A���ӵı����ϵ�һ±����ֻ��2�֣�

(1)A�Ľṹ��ʽ��________��
(2)D��H��M�ķ�Ӧ������________��
(3)A��D�ķ�Ӧ������________��F��G�ķ�Ӧ������________��
A���ɷ�Ӧ
B������Ӧ
C��ȥ��Ӧ
D����Ӧ
(4)I�еĹ����ŵ�������________��
(5)д����ѧ����ʽ��
B��C��________��
C��D��E��________��
M��NaOHˮ��Һ��Ӧ��________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�л���A����C��H��O����Ԫ����ɣ���һ����������A����ת��Ϊ�л���B��C��D��E��ת���ϵ���£�
ij�л���A����C��H��O����Ԫ����ɣ���һ����������A����ת��Ϊ�л���B��C��D��E��ת���ϵ���£�
| ||
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
| ���� |
| ���� |
| ���� |
| �� |
| ���� |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����ͨ�ߵ�ѧУ����ȫ��ͳһ�������ۻ�ѧ���֣��Ĵ����������� ���ͣ���ѡ��
��14�֣���֪����CHO + (C6H5)3P=CH��R �� ��CH=CH��R + (C6H5)3P=O��R����ԭ�ӻ�ԭ���š�W��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH����ϳɷ������£�
���У�M��X��Y��Z�ֱ����һ���л���ϳɹ����е���������ͷ�Ӧ��������ȥ��X��W��һ�������·�Ӧ����������N��N����Է�������Ϊ168��
��ش��������⣺
��1��W�ܷ�����Ӧ�������� ����д��ĸ��ţ�
| A��ȡ����Ӧ | B��ˮ�ⷴӦ | C��������Ӧ | D���ӳɷ�Ӧ |
 Ϊƽ��ṹ����W����������� ��ԭ����ͬһƽ�档
Ϊƽ��ṹ����W����������� ��ԭ����ͬһƽ�档�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���Ĵ�ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
������A����Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ����ͼ��ʾ��

��֪��
a. R�� CH = CHOH���ȶ����ܿ�ת��ΪR�� CH2CHO��
b. R�� CH2CHO���Ժ�����Cu(OH)2��������Һ��������ˮ��R��CH2COOH��Cu2O��
c. H��C��C��H��ԭ����һ��ֱ���ϡ�
����������Ϣ�ش��������⣺
��1��A�ķ���ʽΪ__________________________________________________��
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________________________________��
��3��A�Ľṹ��ʽ��______________________________________________��
��4����Ӧ�ٵĻ�ѧ����ʽ��___________________________________________��
��5��A�ж���ͬ���칹�壬д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ��______________��____________��________________��________________��
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����ͨ�ߵ�ѧУ����ȫ��ͳһ�������ۻ�ѧ���֣��Ĵ��������棩 ���ͣ�ѡ����
��14�֣���֪����CHO + (C6H5)3P=CH��R �� ��CH=CH��R + (C6H5)3P=O��R����ԭ�ӻ�ԭ���š�W��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH����ϳɷ������£�

���У�M��X��Y��Z�ֱ����һ���л���ϳɹ����е���������ͷ�Ӧ��������ȥ��X��W��һ�������·�Ӧ����������N��N����Է�������Ϊ168��
��ش��������⣺
��1��W�ܷ�����Ӧ�������� ����д��ĸ��ţ�
A.ȡ����Ӧ B.ˮ�ⷴӦ C.������Ӧ D.�ӳɷ�Ӧ
��2����֪ Ϊƽ��ṹ����W�����������
��ԭ����ͬһƽ�档
Ϊƽ��ṹ����W�����������
��ԭ����ͬһƽ�档
��3��д��X��W��һ�������·�Ӧ����N�Ļ�ѧ����ʽ ��
��4��д������3��̼ԭ���Ҳ�������X��ͬϵ��Ľṹ��ʽ�� ��
��5��д���ڢڲ���Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
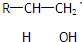
| ||
| �� |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com