
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
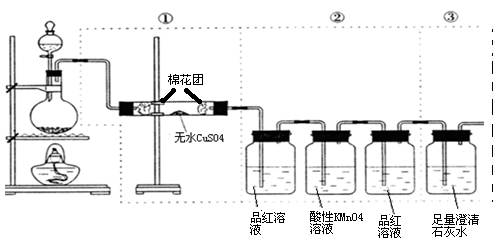
 ��Cu(OH)2��м�ʽ����ͭ���ɱ�ʾΪxCuSO4.yCu(OH)2��.Ϊ�˼�����֤��С���Ա����������ʵ�飺
��Cu(OH)2��м�ʽ����ͭ���ɱ�ʾΪxCuSO4.yCu(OH)2��.Ϊ�˼�����֤��С���Ա����������ʵ�飺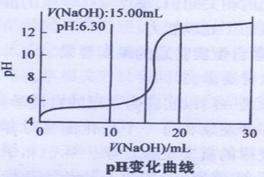
| A����ͷ�ι� | B����ʽ�ζ��� | C����ʽ�ζ��� | D����Ͳ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
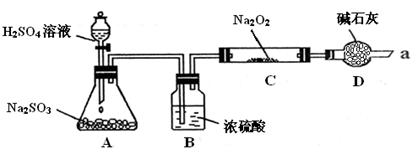

| ʵ�鲽�� | ʵ������ |
| �ٵμ��������ϡ���� | �����ݼ�����ζ���� |
| �ڵμ���������BaCl2��Һ | ������ɫ������ |
| ��ȡ����C�й���������Թ��У���������������ˮ�ܽ� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
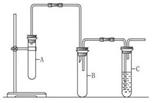
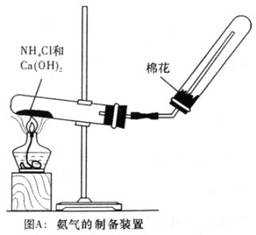
 -���������ѷ�����ȥ��Ӧ��������_______________������ͨ��ʲ
-���������ѷ�����ȥ��Ӧ��������_______________������ͨ��ʲ ôʵ�������֤��________________________________________��
ôʵ�������֤��________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
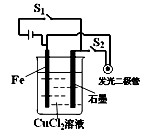
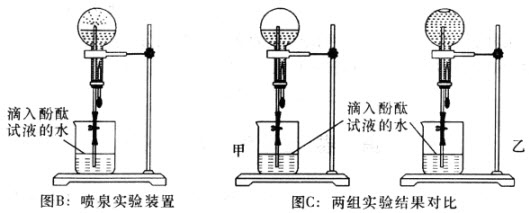
| A���Ͽ�����S1�����¿���S2����ѧ��ת��Ϊ���ܣ�����ת��Ϊ���ܵ� |
| B���Ͽ�����S1�����¿���S2����ʱ���ɵ�װ�����ڵ��� |
| C���Ͽ�����S2�����¿���S1����ʱ���ɵ�װ������ԭ��� |
| D���Ͽ�����S2�����¿���S1����ѧ��ת��Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
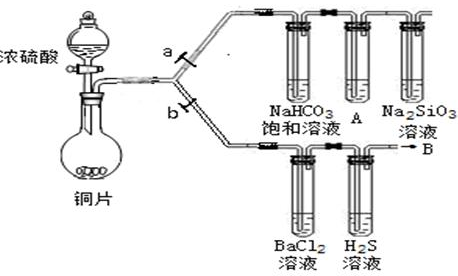
| ʵ����� | ����ͽ��� |
| ����һ��ȡ��ȡ����ϲ���Һ�μ�2��K4[Fe(CN)6] | ��_________�������һ�������� |
| ���������̽����������Һ�м��������� �ѣ���������÷ֲ� | �����Ѳ��Ѫ��ɫ����___________�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
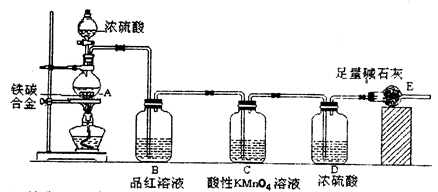
 ������ͬѧ���������װ��������ϴ��ڶദȱ�ݶ�����ʵ������ȷ�����лᵼ����������������ֵƫ�͵��ǣ��δ�һ�����ɣ�
������ͬѧ���������װ��������ϴ��ڶദȱ�ݶ�����ʵ������ȷ�����лᵼ����������������ֵƫ�͵��ǣ��δ�һ�����ɣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com